This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Accelerating the discovery of new materials via the ion-exchange method
Tohoku University researchers have unveiled a new means of predicting how to synthesize new materials via the ion-exchange. Based on computer simulations, the method significantly reduces the time and energy required to explore for inorganic materials.
Details of their research were published in the journal Chemistry of Materials on April 17, 2024.
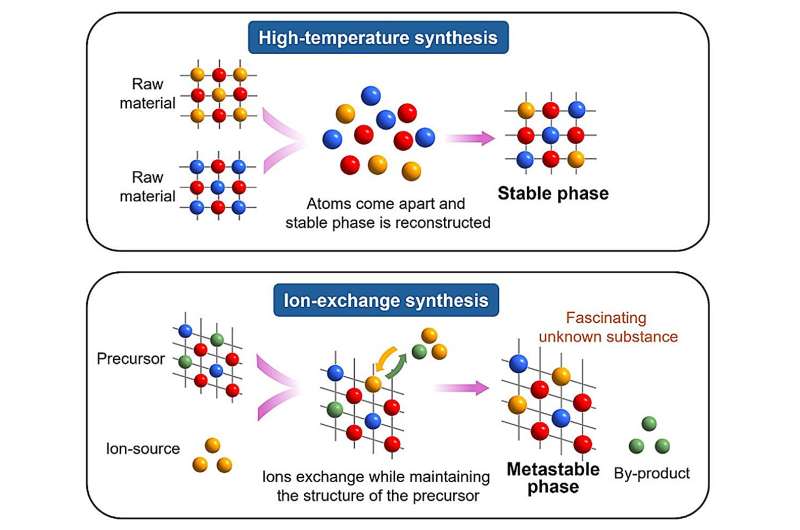
In the quest to form new materials that facilitate environmentally friendly and efficient energy technologies, scientists regularly rely on the high temperature reaction method to synthesize inorganic materials. When the raw substances are mixed and heated to very high temperatures, they are split into atoms and then reassemble into new substances. But this approach has some drawbacks. Only materials with the most energetically stable crystal structure can be formed, and it is not possible to synthesize materials that would decompose at high temperatures.
On the contrary, the ion-exchange method forms new materials at relatively low temperatures. Ions from existing materials are exchanged with ions of similar charge from other materials, thereby forming new inorganic substances. The low synthesis temperature makes it possible to obtain compounds that would not be available by the usual high temperature reaction method.
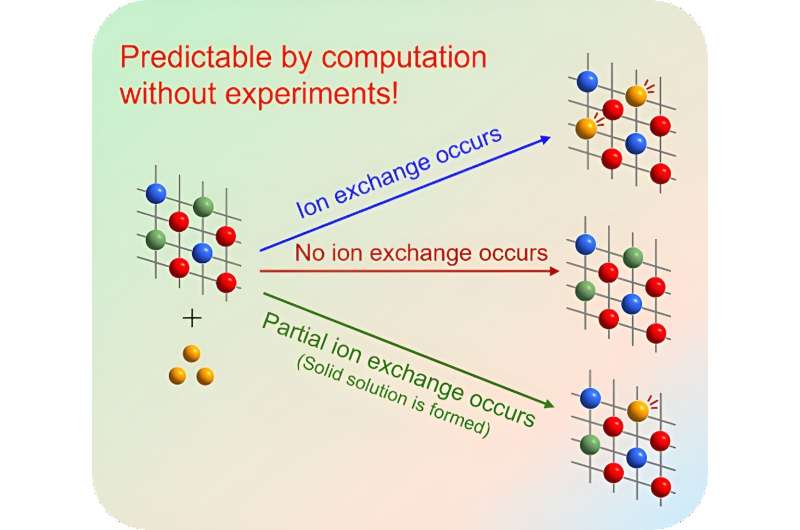
Despite its potential, however, the lack of a systematic approach to predicting appropriate material combinations for ion exchange has hindered its widespread adoption, necessitating laborious trial-and-error experiments.
"In our study, we predicted the feasibility of materials suited for ion exchange using computer simulations," says Issei Suzuki, a senior assistant professor at Tohoku University's Institute of Multidisciplinary Research for Advanced Materials, and co-author of the paper.
The simulations involved investigating the potential for ion exchange reactions between ternary wurtzite-type oxides and halides/nitrates. Specifically, Suzuki and his colleagues performed simulations on 42 combinations of β-MIGaO2, MI = Na, Li, Cu, Ag as precursors, and halides and nitrates as ion sources.
The simulation results were divided into three categories: "ion exchange occurs," "no ion exchange occurs," and "partial ion exchange occurs (solid solution is formed). To confirm their results, the researchers verified the simulation through actual experiments, confirming an agreement between simulation and experiments in all 42 combinations.
Suzuki believes that their advancement will accelerate the development of new materials suitable for improved energy technologies. "Our findings have shown that it is possible to predict whether ion exchange is feasible and to design reactions in advance without experimental trial and error. In the future, we plan to use this method to search for materials with new and attractive properties that will tackle energy problems."
More information: Issei Suzuki et al, Designing Topotactic Ion-Exchange Reactions in Solid-State Oxides Through First-Principles Calculations, Chemistry of Materials (2024). DOI: 10.1021/acs.chemmater.3c03016
Journal information: Chemistry of Materials
Provided by Tohoku University