This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Self-adjusted reaction pathway enables efficient oxidation of aromatic C-H bonds over Co@Y catalyst
The selective oxidation of aromatic C-H bonds has drawn significant attention in both industrial and fine chemistry due to its crucial role in converting readily- and cheaply-available aromatic hydrocarbons into high-value-added oxygenated products for diverse fields such as biology, medicine, fragrance, and agriculture.
The traditional methods of acetophenone (ACP) production suffer from numerous limitations, such as the use of carcinogenic substrates, unrecyclable catalysts, harsh reaction conditions, and low product yields. Through extensive explorations, solvent-free selective ethylbenzene (EB) oxidation with molecular oxygen has been achieved using cobalt naphthenate as a benchmark homogeneous catalyst.
In comparison with homogeneous catalysis, heterogeneous catalysis shows unparalleled advantages in catalyst recycling and product separation and is more suitable for industrial production. Eligible heterogeneous catalysts for EB-to-ACP conversion are being pursued, which remains a key challenge in selective oxidation catalysis.
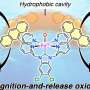
An in situ ligand-protected hydrothermal route was employed to encapsulate cobalt complexes (Co-DETA) in the faujasite matrix. Subsequently, Co@Y catalyst, consisting of cobalt ions confined in zeolite Y, was obtained through the removal of organic ligands upon calcination and was tested for the solvent- and additive-free selective EB oxidation to ACP.
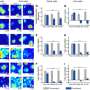
Characterization results from XRD, STEM, UV-vis, and SSNMR reveal that the single-site Co2+ were stably confined in zeolite Y through interaction with framework oxygen atoms, which were further identified as the active sites for EB oxidation. Comparative studies of EB oxidation over various cobalt-containing zeolites and cobalt naphthenate catalysts were conducted to elucidate the crucial role of single-site Co2+ confined in Co@Y.
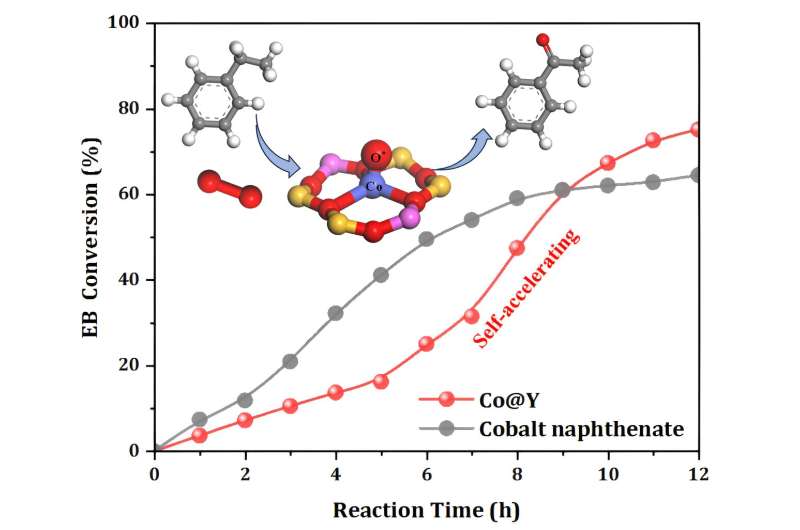
Co@Y exhibited superior performance than other catalysts under identical reaction conditions. The hot filtration test of Co@Y indicates no leaching of Co species during the reaction, confirming the heterogeneous nature of EB oxidation catalyzed by Co@Y. Moreover, after multiple recycles, the structure and catalytic performance of Co@Y remained nearly unchanged, revealing its perfect stability.
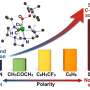
Notably, the self-accelerating phenomenon was observed during the progress of EB oxidation. Comparative tests of EB oxidation with the addition of benzaldehyde or 1-phenylethanol demonstrate that benzaldehyde or 1-phenylethanol can modulate the catalytic behaviors of Co@Y, reducing the apparent activation energy for EB oxidation.
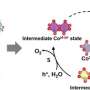
The mechanism of the EB oxidation at the single-site Co2+ in Co@Y catalyst and the origin of the self-acceleration phenomenon were systematically studied by the first-principles density functional theory (DFT) calculations. It is revealed that trace benzaldehyde or 1-phenylethanol generated in the reaction can promote EB oxidation to ACP, bypassing the traditional pathway of ethylbenzene to 1-phenylethanol and finally to acetophenone.
The self-acceleration phenomenon in EB oxidation was attributed to the generation of the reactive oxygen species (O*) at the single-site Co2+, which acted as an 'initiator' to promote the subsequent chain reactions.
In summary, this work from a research group led by Prof. Landong Li from Nankai University, China, provides valuable insights into the experimental phenomena and the catalytic mechanisms involved in the oxidation of aromatic C-H bonds over single-site cobalt catalysts, opening a new avenue for catalyst design.
The results were published in the Chinese Journal of Catalysis.
More information: Jian Dang et al, Self-adjusted reaction pathway enables efficient oxidation of aromatic C–H bonds over zeolite-encaged single-site cobalt catalyst, Chinese Journal of Catalysis (2024). DOI: 10.1016/S1872-2067(23)64579-6
Provided by Chinese Academy of Sciences