This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
In vivo production of CAR-T cells using virus-mimetic fusogenic nanovesicles
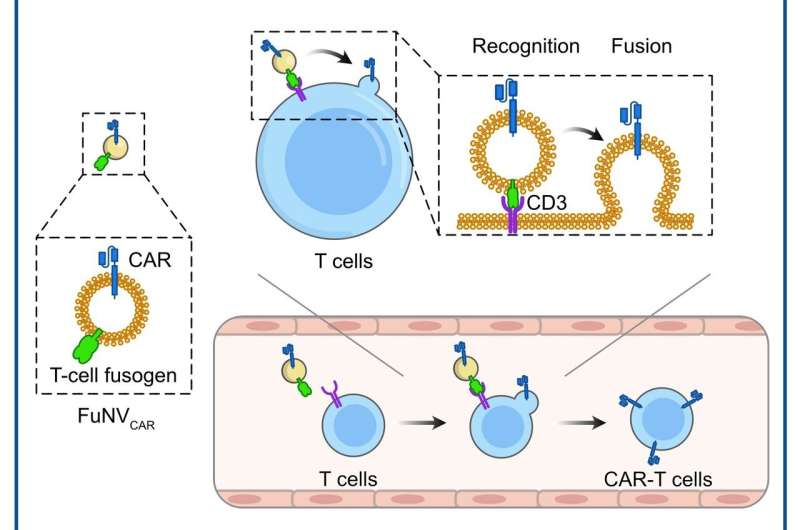
Chimeric antigen receptors (CARs) are synthesized membrane proteins that enable lymphocytes to recognize and respond to the specific antigens of target cells. Despite the impressive efficacy of CAR-T cell therapy in treating B-cell lymphoma or leukemia, the expensive and complex manufacturing process has hindered its widespread clinical application.
Previous research has explored the use of nanoparticles for delivering nucleic acids to program circulating T cells in vivo, streamlining CAR-T cell generation and obviating the need for isolating T cells from patients. Meanwhile, inserting the CAR protein directly into the T cell membrane could present a straightforward method, circumventing complications such as cytokine release syndrome (CRS) and the tumorigenic risk associated with random viral gene insertion into the genome.
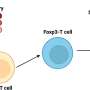
Led by Prof. Jun Wang and Prof. Cong-Fei Xu from the School of Biomedical Sciences and Engineering at the South China University of Technology, researchers have developed a promising strategy that involves the direct fusion of CAR molecules, pre-expressed on fusogenic nanovesicles (FuNVs), to T cells, thereby constructing CAR-T cells in vivo.
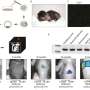
They engineered the T-cell fusogen by adding an anti-CD3 single-chain variable fragment to the reovirus or the measles virus fusogen. They demonstrated that FuNVs derived from T-cell fusogen-expressing cells carried a substantial quantity of T-cell fusogen, which efficiently induced the fusion between NVs and T cells both in vitro and in vivo.
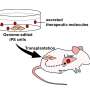
Subsequently, considering the clinical success of anti-CD19 (αCD19) CAR-T cells, the engineered cells expressing T-cell fusogen and αCD19 CAR protein were constructed to produce αCD19 CAR-carrying FuNVs (FuNVCAR). CAR-T cell production was successfully achieved by delivering CAR protein onto T-cells via FuNVCAR in vitro and in vivo. Meanwhile, intravenous injection of FuNVCAR effectively inhibited B-cell lymphoma growth.
To further explore the potential toxicity of FuNVCAR, blood counts and serum biochemical analyses were conducted at 2 days and 14 days, demonstrating comparability to the control group. Throughout the treatment with FuNVCAR, no significant alterations in body weight were observed in mice.
Furthermore, in contrast to traditional CAR-T cell treatment, treatment with FuNVCAR did not induce an elevated release of inflammatory cytokines. This observed difference can be attributed to transient CAR-T cells produced by FuNVCAR, which undergoes limited and temporary activation, mitigating the sustained release of inflammatory cytokines.
In summary, this study introduces a novel approach for in vivo CAR-T cell production through FuNV-mediated CAR protein delivery. It is essential to note, however, that this strategy may not be suitable for patients with compromised T cell function.
The findings are published in the journal Science Bulletin.
More information: Gui Zhao et al, In vivo production of CAR-T cells using virus-mimetic fusogenic nanovesicles, Science Bulletin (2023). DOI: 10.1016/j.scib.2023.11.055
Provided by Science China Press