This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Nanozyme-enabled nanodecoys: A new strategy for fighting urinary tract infections
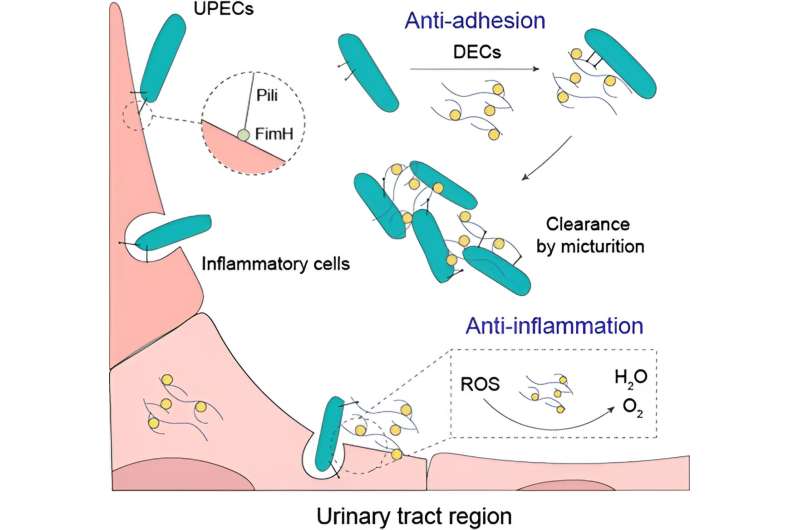
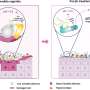
Urinary tract infections (UTIs), affecting millions worldwide, are predominantly caused by uropathogenic Escherichia coli (UPEC). These infections are characterized by bacterial adhesion and colonization in the urinary tract, evading host immune responses. Researchers from Nanjing University have recently reported a new approach to combating UTIs through the development of bioinspired nanozymes acting as nanodecoys.
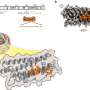
The nanozymes, designed to mimic the function of uromodulin (UMOD), a natural defense mechanism against bacterial invasion, offer a promising solution. By incorporating dextran onto ceria nanoparticles, the nanozymes simulate UMOD's glycans, effectively obstructing the adhesion of UPECs.
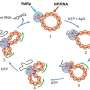
The bioinspired nanozymes exhibit multifaceted functionalities, including anti-inflammatory and anti-adhesive properties, which position them as potential game-changers in UTI treatment. Through their unique design, the nanozymes can scavenge reactive nitrogen and oxygen species (RNOS) generated during infection-induced inflammation.
Additionally, they prevent bacterial adhesion to host cells and abiotic surfaces, reducing bacterial colonization. The nanozymes' ability to alleviate inflammation and mitigate tissue damage offers a comprehensive approach to UTI management.
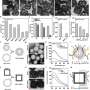
In preclinical studies utilizing mouse models, the nanozymes demonstrated promising results in treating acute UTIs, repeated infections, and catheter-associated UTIs. The bioinspired nanozymes hold significant potential for clinical translation by effectively reducing bacterial colonization and inflammation in the urinary tract.
This innovative approach not only addresses the challenges posed by traditional antibiotic therapies but also offers a safer and more comprehensive strategy for managing UTIs and related complications.
The paper is published in the journal ACS Nano.
More information: Yihong Zhang et al, Bioinspired Nanozymes as Nanodecoys for Urinary Tract Infection Treatment, ACS Nano (2024). DOI: 10.1021/acsnano.3c12783
Journal information: ACS Nano
Provided by Nanjing University