This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Pushing the limit of the periodic table with superheavy elements
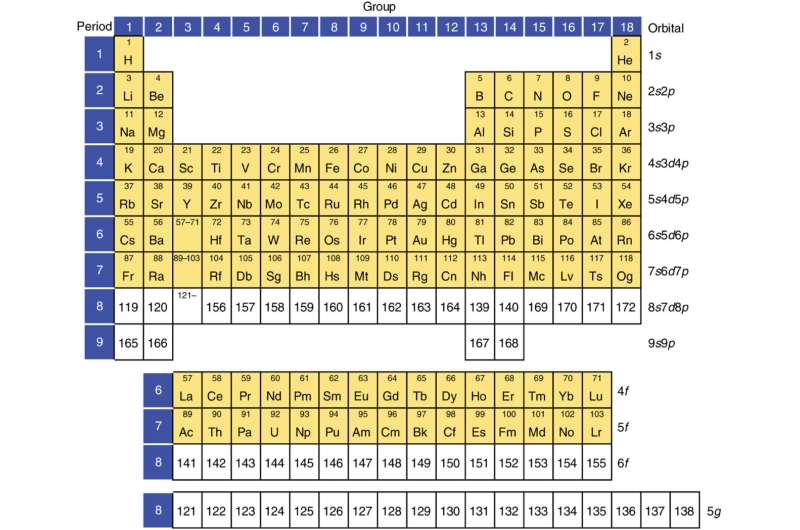
Scientists from Massey University in New Zealand, the University of Mainz in Germany, Sorbonne University in France, and the Facility for Rare Isotope Beams (FRIB) discuss the limit of the periodic table and revising the concept of the "island of stability" with recent advances in superheavy element research. Their work first appeared in Nature Reviews Physics.
In addition to the Nature Reviews Physics feature, Physics Reports has published a review on the atomic electronic structure theory for superheavy elements.
What is the heaviest bound nucleus and the heaviest bound atom and what are their properties? The nuclei of chemical elements with more than 103 protons are labeled as "superheavy." They are part of a vast unknown territory of these nuclei that scientists are trying to uncover. Exploring this uncharted territory provides prospects for discoveries that connect the broad areas of science.
New experimental facilities are being built to help scientists to uncover properties of atoms and their nuclei in a regime of very large numbers of electrons, protons, and neutrons. The facilities will create new elements and nuclides at the limits of atomic number and mass. The production rates of superheavy nuclei are exceedingly low.
The physical and chemical data obtained from these experiments has indicated deviations from lighter elements and isotopes. This allows scientists to question how much further the borders of the Periodic Table of the Elements and the Chart of the Nuclides can be expanded. Assessing the existence of the "peninsula of extended stability," where superheavy nuclei could have lifetimes beyond the very short lived one discovered up to now, is also a scientific goal.
In addition, the progress of atomic structure theory focuses on superheavy elements and their predicted electronic ground state configurations, which are important for an element's placement in the periodic table.
"Due to the presence of huge electrostatic forces, electrons in superheavy atoms move with velocities close to light speed," said one of the authors of the paper, Witek Nazarewicz, John A. Hannah Distinguished Professor of Physics and chief scientist at FRIB. "Also, very strong Coulomb forces in superheavy nuclei give rise to new effects. This is a new ball game for atomic and nuclear theory."
At FRIB, scientists will study ways to reach superheavy nuclei located more closely toward the region of enhanced stability. Many superheavy nuclei cannot be measured currently, so information about them must come from theoretical extrapolations. Nuclear theorists at FRIB carry out predictions for superheavy nuclei using advanced models aided by high-performance computing and machine learning.
Studying the Periodic Table of Elements and the nuclear landscape in the superheavy region will generate new ideas and methods that will impact nuclear and atomic physics, astrophysics, and chemistry.
More information: O.R. Smits et al, Pushing the limits of the periodic table — A review on atomic relativistic electronic structure theory and calculations for the superheavy elements, Physics Reports (2023). DOI: 10.1016/j.physrep.2023.09.004
Journal information: Nature Reviews Physics
Provided by Michigan State University





















