March 25, 2024 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Breaking the ice: Molecular insights into saltwater droplet freezing
In a new study, researchers have observed the freezing of saltwater droplets at a molecular level, offering new insights for de-icing and anti-icing technologies. Contrary to conventional wisdom, these droplets don't conform to the typical freezing patterns observed in pure water.
The research team, whose study was published in Nature Communications, performed experiments to uncover the formation of a brine (saltwater) film on top of the frozen seawater droplets, which had previously not been reported.
This was accompanied by the emergence of ice crystals from the bottom of the brine film, which grow until they pierce the top of the droplet in a phenomenon termed "ice sprouting." These were validated using molecular dynamics (MD) simulations
They further conducted an analogous experiment to measure rates of ice precipitation and condensation, supporting the proposed mechanism.
Pure vs. saltwater freezing
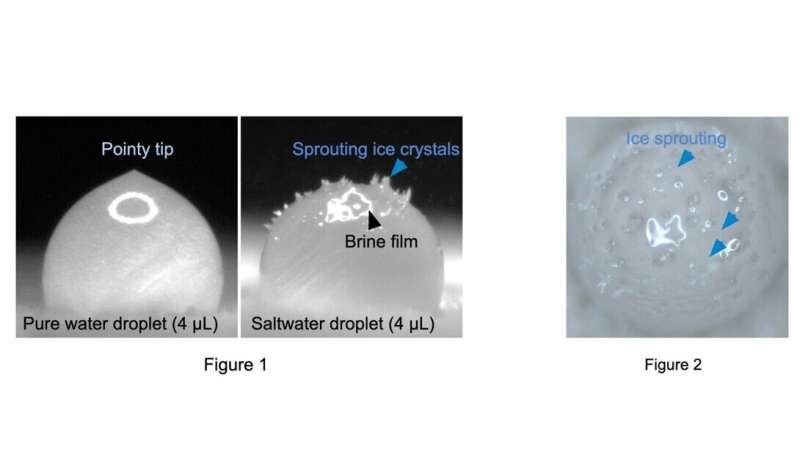
Freezing of pure water droplets typically follows a well-understood process where the droplet gradually cools until it reaches its freezing point. Then, the ice crystals form and grow, taking a solid ice structure with a singular, pointy tip.
On the other hand, the freezing of saltwater droplets introduces additional complexities. As the droplet freezes, the salt concentration inside affects the freezing point, typically lowering it compared to pure water. This also makes the pointy tip of the droplet disappear, as reported in previous research.
The icing process, which refers to the accumulation of ice on surfaces or objects due to the freezing of water droplets, can cause damage to several processes, such as navigation, aviation, and infrastructure.
However, the behavior of saltwater droplets introduces additional considerations. The presence of a brine layer can influence the adhesion of the frozen droplet to surfaces, potentially affecting anti-icing strategies or surface coatings designed to mitigate icing.
The study's first author, Dr. Fuqiang Chu, an Associate Professor at the University of Science and Technology, Beijing, spoke to Phys.org about their work.
"I am curious about the icing phenomenon and began to study it while pursuing my doctoral degree. However, I think people could not fully understand this phenomenon until now, especially when using a binary droplet, such as a salty droplet."
"In this work, we studied the process of salty droplet freezing and tried to discover the uniqueness of salty droplet freezing compared to pure water droplets," said Dr. Chu.
Observing and analyzing the freezing process
To study the freezing process of salt water, the researchers used saltwater with varying salt concentrations. They used a semiconductor refrigeration module to provide controlled cooling, allowing them to tune the surface temperature below the freezing point of the droplets.
Saltwater droplets were injected onto the experimental surface, where they underwent the freezing process. High-speed microphotography was used to record and analyze the icing phenomena, including the liquid film formation on top of frozen droplets.
They observed the presence of concentrated brine within the frozen salty droplets, indicating incomplete freezing, which is different from the freezing of pure water droplets.
Based on temperature measurements, the researchers devised a method to predict the freezing duration of salty droplets. They correlated the appearance of a liquid film on top of frozen droplets with the end of the freezing process, providing a visual indicator for freezing time determination.
The MD simulations were then used to validate and complement the experimental results by offering a molecular-level perspective, allowing researchers to understand the underlying mechanisms driving the observed phenomena.
The MD simulations aimed to reproduce the experimental observations and provide additional insights into the molecular interactions occurring during droplet freezing by simulating the behavior of ions, water molecules, and freezing interfaces at the nanoscale.
Ice sprouting
The researchers observed the formation of a brine layer on top of the frozen droplet. This layer prevents the formation of a pointed tip and maintains a stable temperature within the droplet.
"After the formation of the brine film, some ice crystals begin to sprout at the bottom of the film, which is very similar to the process of seed germination. This ice-sprouting phenomenon surprised me, making me feel like the droplets were living and nurturing a new life," said Dr. Chu.
This unique phenomenon results in the puncturing of the brine film and further ice crystal growth in the air.
The ice sprouting phenomenon is governed by interfacial condensation on the saturated brine film under humid air conditions.
In other words, since the temperature of the brine film is lower than the surrounding air's dew point (the temperature at which air becomes saturated with water vapor), it causes water vapor from the air to condense at the interface of the brine film.
This condensed water dilutes the brine film, disrupting its balance or equilibrium. As a consequence of this dilution, the brine film becomes supersaturated with salt, leading to the precipitation of ice crystals from within the film. The ice crystals that form within the brine film increase its salt concentration, thereby re-saturating the brine film at its temperature.
"This suggests that the environmental humidity effect cannot be ignored when studying the phase transition or crystallization process of solutions," added Dr. Chu.
Universal definition of freezing duration
In addition to these two observed phenomena, the researchers proposed a universal definition of freezing duration for quantifying the icing rate of droplets with varying salt concentrations. This is an important parameter to evaluate the performance of anti-icing surfaces and technologies.
"Using our definition of freezing duration for salty droplets, researchers may be able to quantitatively evaluate the performance of their anti-icing methods against salty droplets. This may be helpful for the development of marine anti-icing technologies," explained Dr. Chu.
Identifying the formation of the brine film on top of frozen droplets provides researchers with a standardized way to mark the end of the freezing process, making it easier to measure and compare droplet freezing behavior.
Speaking of potential applications to anti-icing technologies, Dr. Chu mentions lowering the adhesion of the frozen saltwater droplets.
"For a saltwater droplet, the whole icing process is manifested as random ice crystal growth, and there is concentrated brine remaining in the crevices of ice crystals."
"As a result, the adhesion of frozen saltwater droplets not only depends on the contact area but also relates to the growth orientation of ice dendrites and the distribution of concentrated brine. These can be controlled by tuning the position of nucleation (initial formation) sites to obtain a low ice adhesion," explained Dr. Chu.
More information: Fuqiang Chu et al, Interfacial ice sprouting during salty water droplet freezing, Nature Communications (2024). DOI: 10.1038/s41467-024-46518-y.
Journal information: Nature Communications
© 2024 Science X Network