February 16, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Conducting supercooled droplet experiments to design and engineer superhydrophobic ice-repellent surfaces

Supercooled droplets can typically freeze on surfaces in nature, and have broad-scale influence on industries where they can adversely impact technical efficiency and reliability. Superhydrophobic surfaces are therefore a materials engineering solution to rapidly shed water and reduce ice adhesion to form promising candidates that resist icing.
However, the impact of supercooled droplet freezing and their effects on droplet-substrate interactions as well as resultant applications across ice-phobic surfaces remain to be explored in physics and materials engineering.
In a new report in Nature Physics, Henry Lambley and a research team in mechanical and processing engineering at the ETH Zurich, Switzerland, studied frozen supercooled droplets resting on textured surfaces. They induced freezing by evacuating the surrounding atmosphere and determined the surface properties required to promote ice formation.
The team explored these outcomes by balancing anti-wetting surface forces with those triggered by the freezing phenomena to demonstrate textures rationally designed to promote ice expulsion. Additionally, they considered the complementary processes of freezing at atmospheric pressure and sub-zero temperatures to observe bottom-up ice suffusion. In this way, Lambley and colleagues assembled a rational framework to study ice adhesion of supercooled droplets to design and develop efficient ice-repellent surfaces for broad-scale applications in life sciences and processing industries.
Developing superhydrophobic surfaces for aviation, infrastructure and power transmission
Droplet freezing on surfaces occurs quite commonly in nature, effectively impacting the transportation, construction and power generation industries. Existing approaches to generate ice-repellence in the lab and in practice are resource-intensive, relying on chemicals or on high energy consumption. Researchers therefore aim to create ice-phobic surfaces that delay freezing for sustainable industrial applications, while facilitating a low-adhesion surface for ice already in formation. To accomplish this, they explored the physics of droplet freezing; a two-step process of rapid recalescence, i.e., a temporary rise in temperature during the cooling of a substance, followed by slow crystallization.
The latent heat released during the process can lead to explosive vaporization and levitation, frost halo formation and cascade freezing phenomena. Additionally, volumetric expansion during crystallization can lead to droplets self-peeling and disintegrating. In this work, the researchers studied freezing behavior of super-cooled droplets on superhydrophobic surfaces across a wide-range of temperatures and pressures. The outcomes provide a blueprint to design robust ice-phobic surfaces across the industries of aviation, civil infrastructure and power transmission.
Understanding the dynamics of water droplet-freezing on superhydrophobic surfaces
During the experiments, the team engineered regular arrays of transparent cylindrical micropillar-like textures on polydimethylsiloxane surfaces (a silicone precursor polymer) via soft lithography. Thereafter, they initiated freezing in an environmental chamber at ambient temperatures by exposing the water droplets of a small volume from the Cassie-Baxter wetting state to a dry, low-pressure environment. The cooling method employed during the study favored nucleation at the free surface.
During the study, the scientists focused on dissecting the intrinsic features underlying the droplet freezing process. At first, they observed the progression of the recalescing supercooled water droplets deposited on the superhydrophobic polymer micropillar surfaces; each sequence demonstrated similarities onset. Beginning with nucleation from the free surface and droplet self-deformation, however, the phenomena yielded three outcomes.
The team coined the first outcome as "impalement," in which the droplet compressed downwards to transit from Cassie-Baxter to Wenzel wetting. The other processes showed "expulsion behavior" as the droplets lifted away from the surface leaving a clean substrate. During the final observation, the droplet remained stationary during recalescence and disappeared via scattering at the droplet-air interface due to ice-slush formation. However, with crystallization, the team noted volumetric expansion into the texture for all three processes.
Studying the effects of freezing
The scientists studied the effects of varying the surface texture on the freezing outcomes and explored the angle of nucleation initiated during droplet formation. The outcomes suggested nucleation-induced symmetry breaking of the droplet during recalescence to have contributed to the mechanism of freezing. Existing research also showed how the evaporation rate of a droplet substantially increased during recalescence.
For further analysis of pressure measurements and droplet evaporation modeling, including kinetic and diffusive resistances to vapor transport, the team used miniaturized pressure sensors. They also studied the adhesion force and capillary forces underlying the interactions between a droplet and a substrate. The team showed the validity of the proposed modeling system and the robustness of the observed method by conducting freezing experiments on diverse polymer substrates.
-
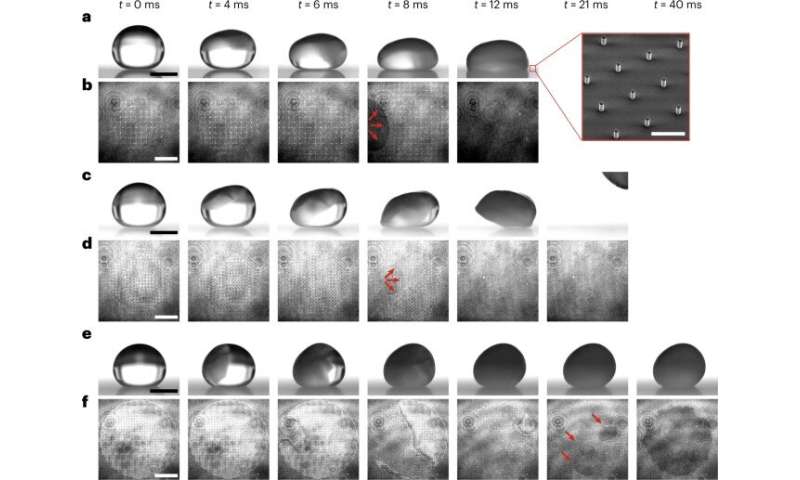
(a–f), Synchronized side- (a,c,e) and bottom- (b,d,f) view image sequences of water droplets freezing through evaporative cooling in a dry, low-pressure environment with different outcomes. (a,b,) Impalement: penetration of the meniscus into the texture characterized by a low final contact angle and full substrate wetting (the dark area in b; red arrows illustrate the spreading direction of the penetrated liquid). The inset in a is a micrograph of the transparent superhydrophobic micropillar surface (scale bar, 100 μm). (c,d), Expulsion: spontaneous de-wetting of the droplet (the receding contact line is marked by red arrows). (e,f), Suffusion: freezing on top of the texture characterized by a high final contact angle, followed by volumetric expansion into the texture (the dark area in f; red arrows indicate the initial regions of substrate wetting). Credit: Nature Physics (2023). DOI: https://doi.org/10.1038/s41567-023-01946-3 -
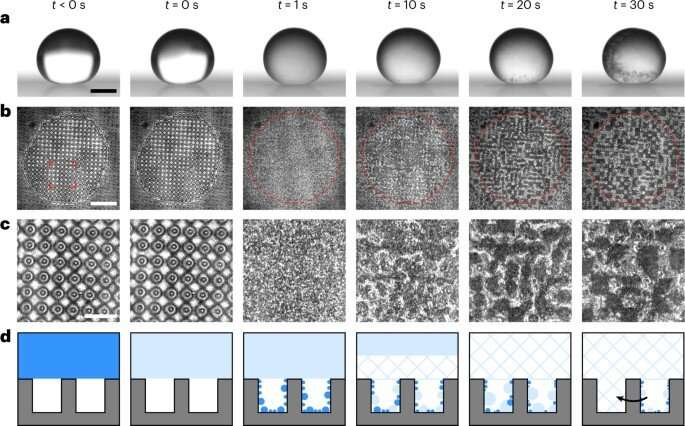
Freezing on superhydrophobic surfaces at ambient pressure. (a,b), Synchronized side- (a) and bottom- (b) view image sequences of a water droplet freezing in a cold, dry environment at atmospheric pressure (red circles mark the approximate location of the contact line post-recalescence) on a superhydrophobic PDMS texture [d, s, h] = [10, 50, 40] µm (identifier D2 in Supplementary Table 1). (c), Enlarged view between the pillars underneath the droplet for each timestep (for the regions of interest marked in b). (d), Schematic of the bottom-up suffusion mechanism responsible for surface failure from condensation filling, coalescence (black arrow) and freezing. Water, ice slush and solid ice are represented by blue shading, light blue shading and hatching, respectively. Scale bars: a, 1 mm; b, 300 µm; c, 100 µm. All experiments (N = 24) showed bottom-up suffusion. Credit: Nature Physics (2023). DOI: https://doi.org/10.1038/s41567-023-01946-3
Outlook
In this way, physicists Henry Lambley and colleagues observed three outcomes of water droplet freezing on superhydrophobic surfaces under low-pressure conditions. They studied the driving and resisting forces underlying the droplets during recalescence to thereby identify a method to design textures and achieve robust droplet expulsion, which they demonstrated with proof-of-concept experiments on hierarchical surfaces. The team revealed the mechanisms of surface anti-icing that required a degree of supercooling to demonstrate the phenomena.
The outcomes have broad-ranging applications in materials engineering to facilitate the development of icephobic surfaces across a large spectrum of environmental pressure and temperature conditions in industries such as aviation, transportation, energy and infrastructure.
More information: Henry Lambley et al, Freezing-induced wetting transitions on superhydrophobic surfaces, Nature Physics (2023). DOI: 10.1038/s41567-023-01946-3
Divya Panchanathan et al, Levitation of fizzy drops, Science Advances (2021). DOI: 10.1126/sciadv.abf0888
Journal information: Science Advances , Nature Physics
© 2023 Science X Network





















