This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Biologists discover the secrets of how gene traits are passed on
A research team has recently made a significant breakthrough in understanding how the DNA copying machine helps pass on epigenetic information to maintain gene traits at each cell division.
Understanding how this coupled mechanism could lead to new treatments for cancer and other epigenetic diseases by targeting specific changes in gene activity. Their findings have recently been published in Nature.
Our bodies are composed of many differentiated cell types. Genetic information is stored within our DNA,which serves as a blueprint guiding the functions and development of our cells. However, not all parts of our DNA are active at all times. In fact, every cell type in our body contains the same DNA, but only specific portions are active, leading to distinct cellular functions.
For example, identical twins share nearly identical genetic material but exhibit variations in physical characteristics, behaviors and disease susceptibility due to the influence of epigenetics. Epigenetics functions as a set of molecular switches that can turn genes on or off without altering the DNA sequence. These switches are influenced by various environmental factors, such as nutrition, stress, lifestyle, and environmental exposures.
In our cells, DNA is organized into chromatin. The nucleosome forms a fundamental repeating unit of chromatin. Each nucleosome consists of approximately 147 base pairs of DNA wrapped around a histone octamer which is composed of two H2A-H2B dimers and one H3-H4 tetramer.
During DNA replication, parental nucleosomes carrying the epigenetic tags, also known as histone modifications, are dismantled and recycled, ensuring the accurate transfer of epigenetic information to new cells during cell division. Errors in this process can alter the epigenetic landscape, gene expression and cell identity, with potential implications for cancer and aging.
Despite extensive research, the molecular mechanism by which epigenetic information is passed down through the DNA copying machine, called the replisome, remains unclear. This knowledge gap is primarily due to the absence of detailed structures that capture the replisome in action when transferring parental histones with epigenetic tags.
Studying the process is challenging because of the fast-paced nature of chromatin replication, as it involves rapid disruption and restoration of nucleosomes to keep up with the swift DNA synthesis.
In previous studies, the research team made significant progress in understanding the DNA copying mechanism, including determining the structures of various replication complexes. These findings laid a solid foundation for the current research on the dynamic process of chromatin duplication.
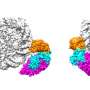
This time, the team achieved another breakthrough by successfully capturing a key snapshot of parental histone transfer at the replication fork. They purified endogenous replisome complexes from early-S-phase yeast cells on a large scale and utilized cryo-electron microscopy (cryo-EM) for visualization.
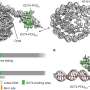
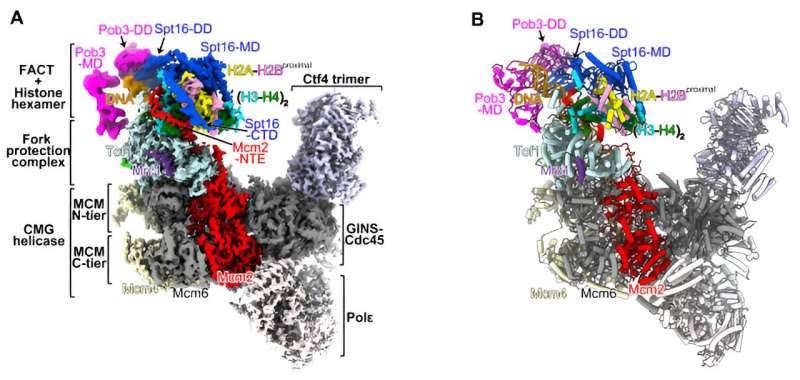
They found that a chaperone complex FACT (consisting of Spt16 and Pob3) interacts with parental histones at the front of the replisome during the replication process. Notably, they observed that Spt16, a component of FACT, captures the histones that have been completely stripped off the duplex DNA from the parental nucleosome. The evicted histones are preserved as a hexamer, with one H2A-H2B dimer missing.
Another protein involved in DNA replication, Mcm2, takes the place of the missing H2A-H2B dimer on the vacant site of the parental histones, placing the FACT-histone complex onto the front bumper of the replisome engine, called Tof1. This strategic positioning of histone hexamer on Tof1 by Mcm2 facilitates the subsequent transfer of parental histones to the newly synthesized DNA strands.
These findings provide crucial insights into the mechanism that regulates parental histone recycling by the replisome to ensure the faithful propagation of epigenetic information at each cell division.
The research was led by Professor Yuanliang Zhai at the School of Biological Sciences, The University of Hong Kong (HKU) collaborating with Professor Ning Gao and Professor Qing Li from Peking University (PKU), as well as Professor Bik-Kwoon Tye from Cornell University.
This study involved a collaborative effort that spanned nearly eight years, starting at HKUST and concluding at HKU. Professor Zhai said, "It only took us less than four months from submission to Nature magazine to the acceptance of our manuscript. The results are incredibly beautiful. Our cryo-EM structures offer the first visual glimpse into how the DNA copying machine and FACT collaborate to transfer parental histone at the replication fork during DNA replication.
"This knowledge is crucial for elucidating how epigenetic information is faithfully maintained and passed on to subsequent generations. But, there is still much to learn. As we venture into uncharted territory, each new development in this field will represent a big step forward for the study of epigenetic inheritance."
The implications of this research extend beyond understanding epigenetic inheritance. Scientists can now explore gene expression regulation, development, and disease with greater depth. Moreover, this breakthrough opens up possibilities for targeted therapeutic interventions and innovative strategies to modulate epigenetic modifications for cancer treatment.
As the scientific community delves deeper into the world of epigenetics, this study represents a major step toward unraveling the complexities of replication-coupled histone recycling.
More information: Ningning Li et al, Parental histone transfer caught at the replication fork, Nature (2024). DOI: 10.1038/s41586-024-07152-2
Journal information: Nature
Provided by The University of Hong Kong