This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Inheritance of parental histones safeguards fate of mouse embryonic stem cells
Researchers led by Prof. Gan Haiyun from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences (CAS) have documented the parental histones inheritance safeguard mechanism, in which parental histone allocation contributes to establishing chromatin states during cell differentiation through altering embryonic stem cell differentiation potential.
The study was published in Nature Genetics.
The process of faithfully transferring and restoring histone posttranslational modifications based on parental histone allocation is central to maintaining cellular identity through cell division.
Recent studies have revealed that replisome proteins Mcm2 and Pole3/Pole4 facilitate parental histone transfer to the replication lagging and leading strand. Whether parental histone allocation is regulated to allow programmed gene expression changes during cellular differentiation is still largely unknown.
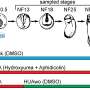
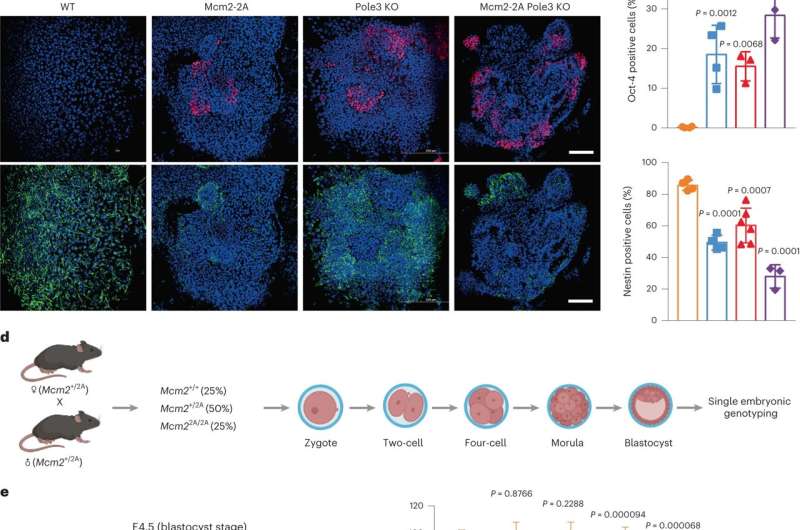
To explore the potential role of parental histone allocation in the process of cell differentiation, the researchers perturbed the symmetric parental histone posttranslational modification inheritance in mouse embryonic stem cells.
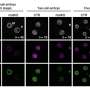
They found that asymmetric parental histone inheritance altered landscapes of histone posttranslational modification H3K27me3. The mutant cells could not fully differentiate along the neural lineage and mouse embryonic development.
Combining single-cell transcriptomics and epigenomics with lineage tracing of cells, they showed that asymmetric parental histone inheritance up-regulated epigenetic and transcriptomic heterogeneity, altering landscapes of histone variant H3.3.
The impaired neural differentiation was caused by a global redistribution of histone posttranslational modifications, especially H3K27me3, which altered the activation of neural lineage genes.
"Our study demonstrates a direct link between DNA replication–coupled histone allocation and cell differentiation," said Prof. Gan.
The last couple of decades have been particularly fruitful for explaining the molecular mechanisms associated with the maintenance of chromatin states during DNA replication. However, a major question had not yet been addressed: to what extent is the inheritance of histone modifications during DNA replication important to control cell identity?
"Gan's lab experimentally assesses this key question in epigenetics. Their study reveals the epigenetic inheritance of histone modifications is involved in the proper establishment of new cell identities, demonstrating that epigenetic control is not a mere mechanism stabilizing established cell states. Rather, it strongly contributes to the dynamic changes of cell fate required for development," commented Pablo Navarro, from Université Paris Cité.
More information: Qing Wen et al, Symmetric inheritance of parental histones contributes to safeguarding the fate of mouse embryonic stem cells during differentiation, Nature Genetics (2023). DOI: 10.1038/s41588-023-01477-w
Journal information: Nature Genetics
Provided by Chinese Academy of Sciences