This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Advancing tissue engineering with shape memory hydrogels
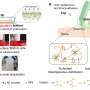
One of the primary goals in the field of tissue engineering and regenerative medicine is the development of artificial scaffolds that can serve as substitutes for damaged tissue. These materials must ideally resemble natural tissue and must have the ability to support cell adhesion, proliferation, and differentiation.
When considering scaffold materials, researchers account for the scaffold's properties, such as its surface roughness, its water content (hydration state), and its flexibility or stiffness (elastic modulus), since these properties are known to affect cell growth.
Hydrogels are biocompatible cross-linked polymers with high water content and are a promising scaffolding material for soft tissue. They can be designed with different elasticities, which can match the mechanical properties of various natural tissues. However, their elastic modulus is linked to their composition, resulting in a difference in the characteristics between softer and harder hydrogels.
To study the specific effect of the hydrogel's elasticity on cell growth, a research team led by Assistant Professor Shin-nosuke Nishimura and Professor Tomoyuki Koga from Doshisha University, Japan, has developed a hydrogel with tunable elastic moduli using the same polymers. The findings of their study were published in the journal Advanced Material Technologies.
"The elastic modulus of hydrogels is one of the most crucial factors in controlling the fate of cells," explains Dr. Nishimura. However, hydrogels with different elasticities are usually prepared by changing the base monomer and the crosslinking agent. This affects not only the elasticity but also various characteristics, such as hydrophilicity and hydrophobicity.
To avoid this problem, the researchers designed the hydrogel without any crosslinks. They used poly(N-acryloylglycinamide) (PNAGAm) as the base polymer, a vinyl polymer with side chains that form strong hydrogen bonds. These bonds break at high temperatures and reattach at lower temperatures, giving these polymers the unique capability to remember and recover their shape in response to temperature changes.
To improve cell adhesion properties of the hydrogel, the researchers combined the PNAGAm polymer with arginine (R)–glycine (G)–aspartic acid (D)–serine (S) peptide through radical copolymerization. These peptides represent the cell-binding sites found in the body and make the hydrogel suitable for cell growth.
Unlike conventional hydrogels, the elastic modulus of the proposed hydrogel can be adjusted by compressing it into different thicknesses at high temperatures.
When exposed to high temperatures, the hydrogen bonds within the polymer break and compressing the hydrogel under such conditions brings the polymer network and hydrogen-based crosslinks closer together. This change in the molecular structure leads to a modification of the hydrogel's elastic modulus.
Upon cooling, due to the reattachment of the hydrogen bonds, the hydrogel maintains both its shape and elastic modulus.
By using this method, the researchers successfully changed the elastic modulus of a rectangular hydrogel bar. They compressed different sections of the hydrogel to thicknesses of 1 mm, 0.64 mm, and 0.50 mm at 65°C for an hour. On cooling it to a cell-culturing temperature of 37°C, the non-pressed, moderately pressed, and firmly pressed regions had elastic moduli of 9,460 Pa, 5,940 Pa, and 3,460 Pa, respectively.
On seeding the hydrogel with mouse embryo fibroblast (NIH/3T3) cells, the researchers observed a direct correlation between the elastic modulus of the hydrogel and the number of adhered cells. In the unpressed region, the number of adhered cells was 1.3 × 104 cells cm−2, whereas in the firmly pressed region, it increased to 1.9 × 104 cells cm−2.
"In this study, we have succeeded in controlling cell adhesion behavior for the first time in the world by utilizing the shape memory properties of hydrogels," says Prof. Koga.
In conclusion, by altering the elastic modulus while keeping other properties consistent, the researchers created a platform which can be used to investigate the influence of elastic modulus on cell growth. This can lead to improved scaffolding materials for tissue regeneration.
More information: Shin‐nosuke Nishimura et al, Regulation of Cell Adhesion on Physically Crosslinked Hydrogels Composed of Amino Acid‐Based Polymers by Changing Elastic Modulus Using Shape Fix/Memory Properties, Advanced Materials Technologies (2024). DOI: 10.1002/admt.202301598
Journal information: Advanced Materials Technologies
Provided by Doshisha University