This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
SPNS2 found to be directly exporting S1P for signaling, can be inhibited
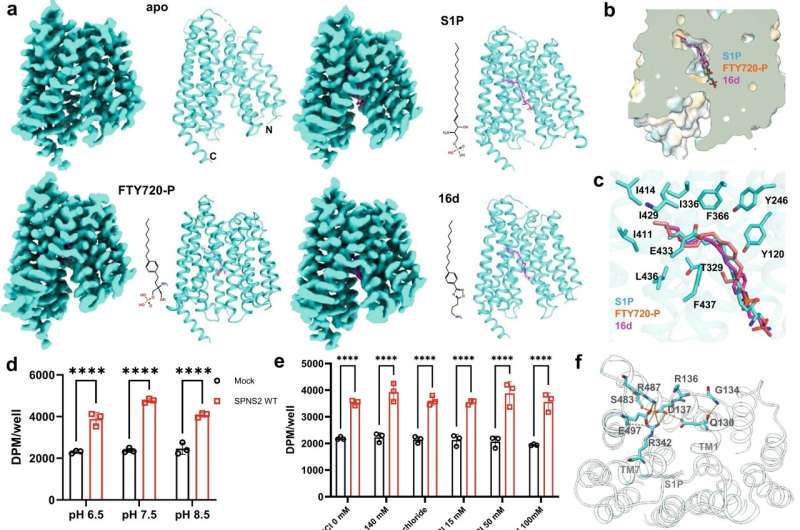
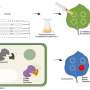
When an enemy invades, defenders are ferried to the site to neutralize the marauders. In the human body, a protein carrier called SPNS2 transports S1P molecules from endothelial cells to rally immune cell response in infected organs and tissues.
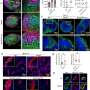
Using specially developed nanobodies that bind to SPNS2 and enlarge the entire structure, the enlarged SPNS2 structure allows the S1P molecules to be viewed via cryogenic electron microscopy. Scientists from the Immunology Translational Research Programme at the Yong Loo Lin School of Medicine, National University of Singapore, and partners have analyzed the structure of the SPNS2 protein at an atomic level that could provide greater insights into how S1P signaling molecules are released to communicate with the immune cells to regulate inflammatory responses.
"Seeing is believing. This work shows that SPNS2 is directly exporting S1P for signaling and it is possible to inhibit its transport function with small molecules. This work provides the foundation for understanding how S1P is released by SPNS2 and how this protein function is inhibited by small molecules for the treatment of inflammatory diseases," said team leader Dr. Nguyen Nam Long.
The SPNS2 protein allows the binding of the S1P signaling molecules to trigger the immune cells to leave the lymph nodes and induce inflammation in different parts of the body when needed. Made up of amino acids, the SPNS2 protein is malleable enough to change its shape and structure to release the S1P signaling molecules through small cavities found within the protein.
Through the discovery of how the SPNS2 protein releases S1P molecules, the SPNS2 structure can be exploited for future drug development. Similar to discovering how the shape of the lock looks like before the key can be designed, this finding sheds more light on how future drugs can be designed to target the protein better to increase drug efficacy.
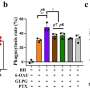
This finding builds on previous research, which found that deleting SPNS2 protein from a pre-clinical model effectively blocks the S1P signaling pathway so that the S1P signaling molecules are unable to be transported to prompt immune cells to leave the lymph node to induce inflammation. Both SPNS2 protein and S1P signaling molecule are required for immune cell recruitment to inflammatory organs, which goes towards treating various inflammatory diseases.
"Using pre-clinical models, we have shown that targeting SPNS2 proteins in the body blocks inflammatory responses in disease conditions, such as multiple sclerosis. This work has provided us a possibility to inhibit its transport function with small molecules that will go a long way to treating inflammatory diseases more efficiently and effectively," said Dr. Nguyen.
The paper was published in Cell Research in December 2023.
More information: Yaning Duan et al, Structural basis of Sphingosine-1-phosphate transport via human SPNS2, Cell Research (2023). DOI: 10.1038/s41422-023-00913-0
Provided by National University of Singapore