This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
The surprisingly simple recipe for starting to grow a limb
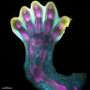
How do organisms form limbs in the womb? Scientists have been striving to answer this question not only to deepen our understanding of evolution and embryonic development, but also to help make the dream of regenerating partial or entire limbs a reality.
A team led by Harvard Medical School geneticists just took a step forward on that long road.
As described in their article published Feb. 5 in Developmental Cell, the researchers identified the special ingredients needed to kick off limb creation in mice and chicks.
"People in the field have known a lot of the proteins critical for limb formation, but we found that there are proteins we missed," said study co-first author ChangHee Lee, research fellow in genetics in the lab of Cliff Tabin at HMS.
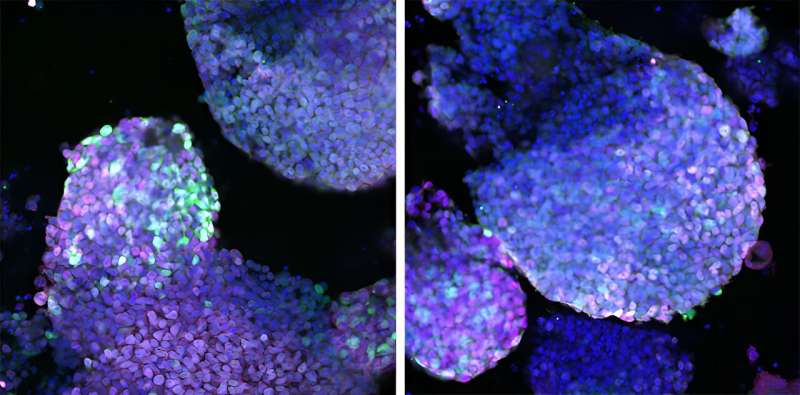
The team found that a combination of just three proteins—Prdm16, Zbtb16, and Lin28a—is necessary and sufficient to turn certain non-limb-forming stem cells into limb-forming ones. A fourth protein, Lin41, speeds the process along.
Part of a family called gene transcription factors, these proteins activate a handful of genes inside certain cells in embryonic tissue known as mesenchyme, the researchers revealed. This change in gene activity is what transforms the cells into limb progenitor cells, the team showed.
Limb progenitor cells then bud out where a limb will form and provide a framework for the future arm, leg, wing, or fin.
"We've found the proteins that imbue 'limbness' to this subgroup of mesenchymal cells," said Lee. "People didn't know how to make mesenchymal stem cells into limb progenitors before. Now we can do this and study early limb differentiation."
Future work needs to confirm whether the same transcription factors are at play in human development. Early work is promising, the team said.
It also remains to be discovered which other ingredients need to be added for limb progenitor cells to mature into the limb's connective tissues, such as tendons, ligaments, and the middle layer of skin.
How the work advances stem cell research
The discovery makes it possible for the first time for scientists to take mouse fibroblasts—connective tissue cells commonly used to explore how stem cells mature into different tissues—and direct them to become limb progenitors.
The work also now allows scientists to keep limb progenitor cells alive in the lab for far longer than was possible before—weeks instead of a day or two. That's enough time to start really digging into the mechanisms of early limb development, Lee said.
Members of the Tabin lab made all of this possible by building a tool to grow limb progenitor cells in 3D structures and then optimizing nearly 30 cell-culture conditions until the cells thrived.
The team was delighted to finally make limb progenitor cells "survive, proliferate, and, critically, maintain their limb progenitor identity after extended culture," said co-senior author Tabin, the George Jacob and Jacqueline Hazel Leder Professor of Genetics and head of the Department of Genetics in the Blavatnik Institute at HMS.
The set of optimal growth parameters is a more important contribution to the field than the 3D scaffold, Lee said. The team made the protocols available for free online.
"We tested a lot of conditions to see what the cells like and what they don't like. We found they are particularly finicky about stiffness," said Lee. "The only limitation we've found so far is that the cells grow so well that they fill up the containers we use, which is a good problem to have."
Questions that limb development studies could now answer
Developmental and evolutionary biologists and regenerative medicine scientists are now better positioned to answer questions such as:
- The roles the three gene transcription factors play in other organ systems and organisms.
- What factors contribute to later limb development, such as fingers and toes.
- What distinguishes fore- and hind limb development.
- How these insights can inform efforts to regrow different organs to treat injury or disease.
"It's important to understand the basic properties of cells that have a therapeutic value," said Lee. "Culturing and maintaining limb progenitor cells and directing them to more specific lineages is fundamentally important for the long-term goal of replenishing cells in the clinic."
The work also supports an underdog argument that mammals can serve as useful model organisms for limb regeneration even though they can't regrow limbs after birth.
"Understanding and harnessing mammalian limb progenitors is a first step toward considering mammals as models for regenerating amputated limbs, as an alternative to the amphibians and other limb-regenerating critters being studied today," said Tabin.
More information: Yuji Atsuta et al, Direct reprogramming of non-limb fibroblasts to cells with properties of limb progenitors, Developmental Cell (2024). DOI: 10.1016/j.devcel.2023.12.010
Journal information: Developmental Cell
Provided by Harvard Medical School