This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researchers reveal how cells regenerate protein factories at the endoplasmic reticulum
The synthesis of proteins in the cell is a key process of life. By this means, the genetic code of the genome is translated into the amino acid sequence of proteins. The process is complex—and has been studied in detail for decades.
Protein biosynthesis is performed by special molecular machines, ribosomes, which consist of a large and small subunit. At the end of protein biosynthesis, these protein factories have to be broken up into their individual parts (recycled), so that they are ready for the next round of translation.
Now a team led by Professor Roland Beckmann, Dr. Thomas Becker, and Ivan Penchev from LMU's Gene Center Munich, working in collaboration with researchers at Stanford University led by Professor Ron Kopito, have shown how the recycling of ribosomes at the so-called endoplasmic reticulum (ER) functions.
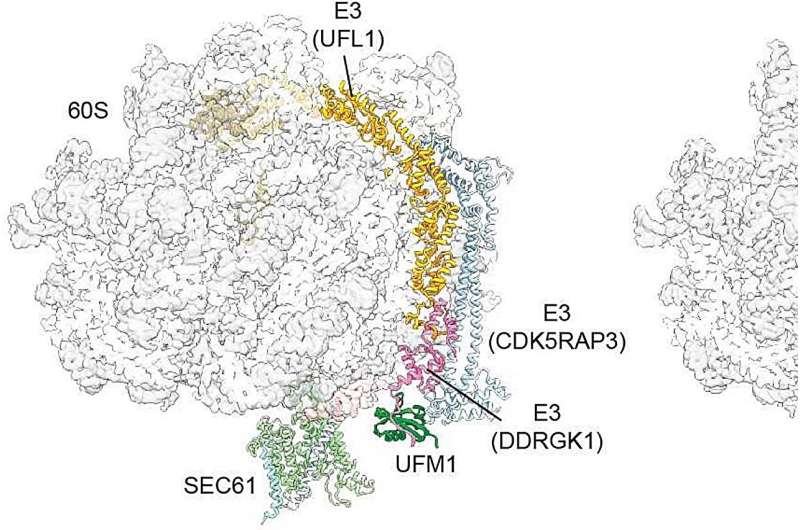
In the process, they discovered the role of an enzyme, a special E3 ligase that joins a small protein modification called UFM1 to the large ribosomal subunit, as a key mechanism of recycling. An account of their investigations has been published in Nature.
Detailed insights into the recycling of ribosomes
Ribosomes are usually found floating within the cytoplasm. "Here we know precisely how the recycling works," says Becker. Sometimes, however, they are located at the ER a continuous cell-wide membrane network.
Although many proteins originate in the cytosol, they subsequently have to be brought to other organelles, such as mitochondria, chloroplasts, and many more. If a protein is synthesized at the ER membrane, the whole translation machinery docks to the ER membrane. This is accomplished with the aid of a protein-conducting channel (SEC61), which is able to transport proteins across the membrane or insert them into the membrane during synthesis.
After completion of the translation, there is a further recycling step, which is specific to the ER membrane. Namely, the large subunit of the ribosome has to be detached again from the protein-conducting channel.
Beckmann's team has now demonstrated how this substep works: When the translation has finished, the E3 ligase recognizes the large subunit of the ribosomes. "It places—figuratively speaking—a small wedge, the protein UFM1, at the large subunit," explains Becker.
"This produces a stable complex out of the modified 60S subunit and the E3 ligase. Simultaneously, it causes the large subunit to detach from SEC61. This is a very important step to ensure that the large subunit is returned to the cytosol and available for the next round."
More information: Paul A. DaRosa et al, UFM1 E3 ligase promotes recycling of 60S ribosomal subunits from the ER, Nature (2024). DOI: 10.1038/s41586-024-07073-0
Provided by Ludwig Maximilian University of Munich