This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researcher proposes paradigm shift in enzyme biochemistry
Although you may never have heard of the cytochrome P450 superfamily of enzymes, these proteins play diverse and critical roles in humans through the metabolic processing of drugs, pesticides, fatty acids, fat-soluble vitamins, and chemical carcinogens and the biosynthesis of essential steroids, including sterols.
Sterols are a family of chemical compounds that share a central, ringed structure and that are critical to the lives of a multitude of organisms. The best-known sterol in humans is cholesterol, a key component of our cell membrane and an ever-present item on physicians' minds considering that elevated blood cholesterol levels can increase our risk of cardiovascular disease.
The laboratory of Fred Guengerich, the Tadashi Inagami, Ph.D. Professor of Biochemistry at Vanderbilt University, has studied cytochromes P450 for 50 years. In a new paper published in Angewandte Chemie, the Guengerich lab probed the mechanism used by cytochrome P450 51—a P450 enzyme present in all families of life—to catalyze a critical, three-step reaction in sterol biosynthesis: the metabolism of lanosterol.
"This has been a challenging but rewarding project that provides the first unambiguous answer to a long-standing and controversial mechanistic question in eukaryotic sterol biosynthesis," said lead author and biochemistry graduate student Kevin McCarty.
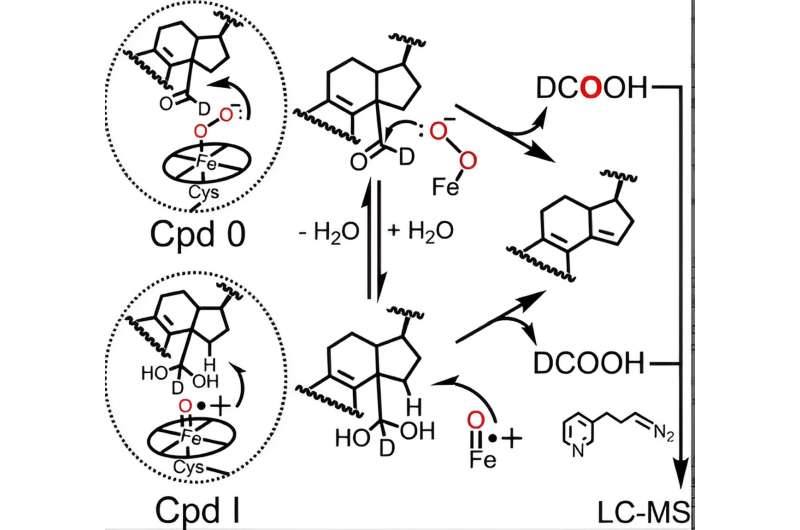
The catalytic cycle of all P450 enzymes involves the formation of two active heme iron species—Compound 0 and Compound I, the latter of which is naturally formed from Compound 0—that are necessary for P450-catalyzed reactions, including lanosterol metabolism. Although the role of Compound I in the first two steps of lanosterol metabolism has been well established, conflicting data from various labs has left scientists unclear about whether P450 51 uses Compound 0 or Compound I to accomplish the crucial final step.
By using an advanced analytical technique initially refined by former Guengerich postdoc Francis Yoshimoto that tracks the incorporation of an oxygen isotope called 18O into the products of the P450 reaction, McCarty and colleagues have become the first to suggest that both Compound 0 and Compound I can play active chemical roles in the last step of lanosterol metabolism.
Indeed, results presented in the Angewandte Chemie paper indicate that while Compound 0 is the major heme species responsible for the last step of human P450 51's catalytic action (~85% of the reaction), Compound I still plays a minor, quantifiable role (~14% of the reaction).
In collaboration with Galina Lepesheva, research professor of biochemistry, the researchers compared the relative contributions of each heme species in four P450 51 enzymes from pathogenic yeast, amoeba, and trypanosomes, a type of parasite, to the human ortholog. While the yeast and amoeba enzymes showed similar results to the human protein, the results from the trypanosomal enzymes revealed an interesting mechanistic difference: Compound 0 and Compound I shared roughly equal contributions to the reaction.
These results add depth to our collective and mechanistic understanding of P450 enzymes, specifically those involved in sterol biosynthesis.
"This was a long project that required a 17-step chemical synthesis, five different purified P450 51 enzymes from our collaborator Prof. Galina Lepesheva, very careful attention to using an 18-oxygen atmosphere in the reactions, sophisticated high-resolution mass spectrometry, and careful work by all the authors in our lab," Guengerich said. According to him, his team's attention to detail allowed it to "crack this system" and provide a clear analysis of a bifurcated enzyme mechanism.
"Our findings provide an important advance in the understanding of P450 51 function in human and various pathogens, which we hope will be useful in the continued search for P450 51–targeted drugs," McCarty said.
Currently, a number of existing antifungal drugs inhibit fungal P450 51 enzymes to interfere with the organism's ability to make essential sterols and reproduce. Yet, resistance to antifungals, coupled with the existence of life-threatening fungal infections for which there is no treatment, underscores the continued need for novel P450 51–targeted drugs.
Looking forward, the Guengerich and Lepesheva labs will further analyze a P450 51 enzyme from amoeba in search of mechanistic peculiarities that may be exploitable as potential drug targets.
More information: Kevin D. McCarty et al, Oxygen‐18 Labeling Reveals a Mixed Fe−O Mechanism in the Last Step of Cytochrome P450 51 Sterol 14α‐Demethylation, Angewandte Chemie International Edition (2024). DOI: 10.1002/anie.202317711
Provided by Vanderbilt University