This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researchers discover way to bind nanotubes to metals
Carbon nanotubes have shown promise for everything from microelectronics to aviation to energy storage. Researchers think this material might one day fulfill the science fiction dream of creating an elevator to space.
So why aren't they used more often?
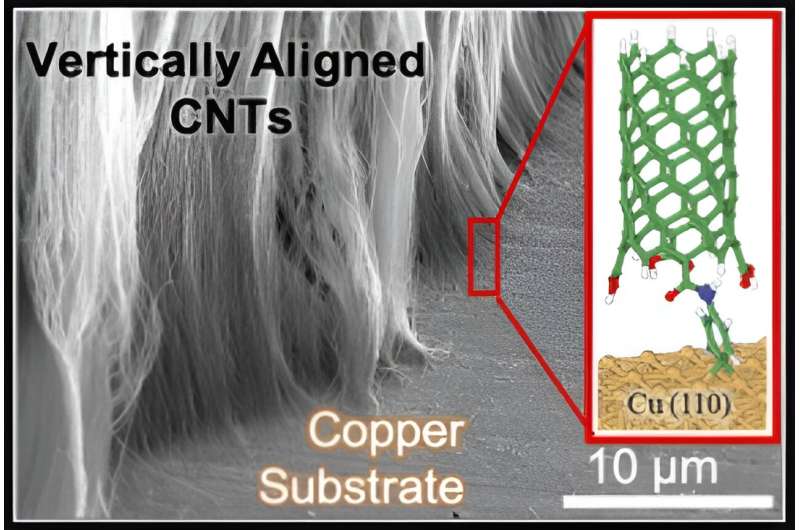
University of Cincinnati chemist Noe Alvarez said one obstacle has been the frustrating inability to link carbon nanotubes to metal surfaces in a robust connection for sensors, transistors and other uses. These hollow tubes have a diameter of just a billionth of a meter but can be many centimeters long.
"We want our experiments to be reproducible and consistent, but that's not easily possible with nanotubes because we can't control how well they're connected to metal surfaces," he said.
But he and his collaborators have demonstrated a new chemical process that grafts nanotubes to metal surfaces to create a strong, consistent, conductive link. The study was published in the journal Nanoscale Advances.
In past iterations, carbon nanotubes were dispersed in a solution to make what Alvarez likens to "wet spaghetti" that sticks to a metal surface.
"But there is no robust connection. Nothing is really holding the nanotubes to the surface," he said.
So measurements of properties such as electrical conductivity were imprecise and inconsistent.
Alvarez and his research partners at Texas A&M University, led by chemical engineering Professor Jorge Seminario, demonstrated ways to bond nanotubes chemically to copper, aluminum, gold and other metal surfaces.
Alvarez and his collaborators received a $720,000 grant from the National Science Foundation to elaborate on their chemical discovery in the next three years.
"Why don't we see carbon nanotubes in widespread commercial applications even though they have so much potential? We have a lot to figure out," UC doctoral student and study lead author Chaminda Nawarathne said.
Alvarez and his co-authors discovered through computational calculations that carbon atoms in the organic link actually bond with two copper atoms, creating an especially strong bond.
"That explains why our nanotubes once they're chemically connected stay connected," Alvarez said.
Carbon nanotubes are notoriously strong molecules. Their molecular structure creates an elegant hexagonal lattice. "Carbon bonds are the strongest bonds. They're covalent bonds. That's why diamond is the hardest material because they are carbon-carbon bonds," Alvarez said.
While carbon atoms in diamonds are single bonds, carbon nanotubes have conjugated double-bonded atoms, making them even stronger than diamonds.
Cables made from strong but lightweight carbon nanotubes have been envisioned for creating "space elevators" that could ferry equipment into orbit, Alvarez said. A space elevator was depicted in the opening scene of the Brad Pitt movie "Ad Astra."
But strength is just one of their unique properties.
Carbon nanotubes are used to create the blackest synthetic material on Earth. Alvarez said their strong bonds with metal could lead to better paints and coatings.
"Nanotubes are fairly inert. They're very stable. You can conjugate them without breaking their bonds. Semiconducting nanotubes also have fluorescence properties—they can generate light," Alvarez said. "So the list of applications goes on and on."
Nawarathne said he is pursuing potential applications in energy storage.
"Now that we can bond the carbon nanotubes to a current collector or metal probe, we can make very stable electrodes for supercapacitors," Nawarathne said.
UC chemistry students "grow" nanotubes on silicon disks using a process called catalytic chemical vapor deposition in equipment that heats reagents and an iron catalyst to 1,450°F.
"It's red-hot," Alvarez said, pointing to an object visible through a glass window in the oven-sized machine. "That's like a baking pan. The catalyst goes in here."
After 45 minutes, a thin layer of carbon nanotubes appears on the silicon. From there, researchers were able to electrograft the nanotubes onto a variety of metal surfaces. Initially, they used bundles of nanotubes but with refined processes learned they can connect vertically aligned nanotubes.
"It's like trying to connect wool back to a sheep. You have yarn that has been sheared from the sheep. We're able to connect individual fibers back to the sheep chemically," he said.
More information: Chaminda P. Nawarathne et al, Creating covalent bonds between Cu and C at the interface of metal/open-ended carbon nanotubes, Nanoscale Advances (2023). DOI: 10.1039/D3NA00500C
Provided by University of Cincinnati