This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chemists create an emission molecular thermometer
Future technologies rely on phenomena that were previously considered the exclusive domain of theoretical physics or chemistry. For example, the approach to devices with high-density information storage arose when chemists discovered single-molecule magnets—unusual complexes of transition metals and lanthanides. In addition, several lanthanide compounds exhibit temperature-dependent luminescence properties.
These properties underlie the creation of molecular thermometers—devices that allow remote temperature measurement with high sensitivity and resolution. These lanthanide thermometers are very promising for future applications in biology, medicine, and cryogenics.
RUDN University chemists have now obtained framework compounds of lanthanides that simultaneously exhibit the properties of monomolecular magnets and lanthanide thermometers. The study is published in RSC Advances.
"Lanthanide compounds of various compositions and structures are of interest to researchers due to their magnetic and luminescent properties. This is impressive not only from a fundamental point of view but also due to its promising technological applications.
"Our goal was to create luminescent compounds with a combination of the properties of emission thermometers and monomolecular magnets. Examples of such compounds are very rare, while they are of particular interest due to the possibility of monitoring the temperature of future monomolecular magnetic devices at the molecular level," Alexey Bilyachenko, Ph.D., a leading researcher at the Joint Institute of Chemical Research of RUDN University said.
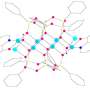
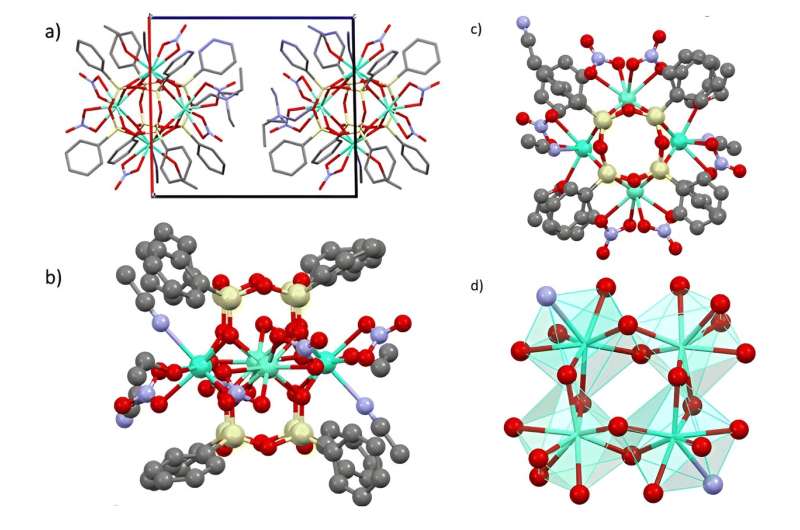
Chemists have synthesized compounds based on silsesquioxanes, an organosilicon matrix that enables framework structures with lanthanide ions of various natures. This essential feature of the frameworks allows, one to obtain behavior that combines the properties of different lanthanide centers. The first three examples of such mixed-metal frames were obtained. They all contain four lanthanide ions.
A compound combining dysprosium and terbium ions (as well as a compound containing a triple combination—europium-terbium-yttrium) exhibits both paramagnetic properties (interaction with a magnetic field) and emission (glow characteristic of these lanthanides).
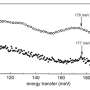
The compound containing europium and dysprosium ions turned out to be the most interesting. It simultaneously exhibits the properties of a monomolecular magnet and a lanthanide thermometer. The compound is applicable as an emission thermometer in a wide temperature range from 20℃ to 100℃ and is characterized by high relative thermal sensitivity (1.15% K-1 at 293 K). The preparation of this compound opens up broad possibilities for the molecular design of functional lanthanide complexes.
"The complex we obtained exhibits unusual, multifunctional properties—a single-molecule magnet and an emission thermometer with a self-calibration mechanism. Further research in this area is very promising for the creation of new generation materials," said Alexey Bilyachenko, Ph.D., a leading researcher at the Joint Institute of Chemical Research of RUDN University.
More information: Gautier Félix et al, Tetranuclear lanthanide-based silsesquioxanes: towards a combination of a slow relaxation of the magnetization and a luminescent thermometry, RSC Advances (2023). DOI: 10.1039/D3RA04901A
Journal information: RSC Advances
Provided by RUDN University