This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists make COVID receptor protein in mouse cells
A team of scientists at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory and Columbia University has demonstrated a way to produce large quantities of the receptor that SARS-CoV-2, the virus that causes COVID-19, binds to on the surface of human cells. That binding between the now-infamous viral spike protein and the human "ACE2" receptor is the first step of infection by the virus. Making functional human ACE2 protein in mouse cells gives scientists a new way to study these receptors and potentially put them to use. In addition, as described in a paper just published in the journal Virology, the method could facilitate the study of other complex proteins that have proven difficult to produce by other means.
The Brookhaven scientists' initial goal, early in the pandemic, was to make large amounts of human ACE2 and then attach the protein to nanoparticles. The ACE2-coated nanoparticles could then be tested as anti-viral therapeutics and/or as sensors for detecting virus particles.
"For either of these applications, you need large quantities of protein, and the protein has to be fully functional," said Brookhaven Lab virologist Paul Freimuth, who led the research in collaboration with scientists at Brookhaven Lab's Center for Functional Nanomaterials (CFN). "But making functional membrane proteins like ACE2 is particularly challenging because the process by which proteins are localized in the cell membrane is complex."
One reason is that these proteins are modified in various ways after they are synthesized and before they are inserted into the cell membrane. In particular, carbohydrate molecules added to the proteins play key roles in both how the long protein chain gets folded into its final 3D structure and how the protein functions in the membrane.
"Carbohydrates account for about one-third of the mass of ACE2 protein," Freimuth said.
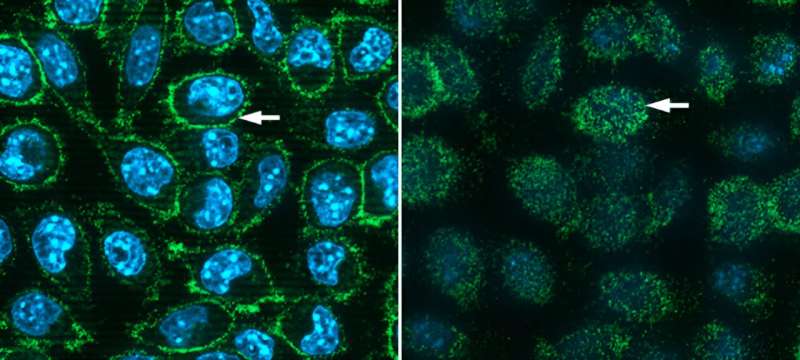
The simplest cells scientists use to generate proteins artificially, namely bacteria, lack the enzymes for attaching those carbohydrate add-ons. So, the Brookhaven team turned to cells from mice, which, as mammals, are more like us—and are thus able to do the same kind of carbohydrate processing. Mouse cells are known to be adept at picking up and expressing "foreign" genes. And while mouse cells also make an ACE2 receptor, the mouse version of the protein doesn't bind to the SARS-CoV-2 spike. That means the scientists would have an easy way to see if the mouse cells made the human ACE2 protein—by seeing if the spikes would bind to the cells.
Finding and expressing the ACE2 gene
To increase the chances that mouse cells would incorporate and read the human ACE2 gene correctly, the group used the intact gene. Genes from humans and other "higher organisms" contain lots of information in addition to the sequence of DNA that codes for the amino acid building blocks that make up a protein. This extra information helps to regulate gene structure and function within the cell's chromosomes.
The scientists searched libraries of cloned DNA fragments that were generated as part of the Human Genome Project—a DOE-sponsored effort to map out the locations of all the genes that make us human—to find a fragment that contained the intact ACE2 gene, complete with its embedded regulatory information. Then, they exposed mouse cells to nanoparticles coated with this DNA fragment plus the gene for another protein that makes cells resistant to a lethal antibiotic.
"In this case, the nanoparticles serve as a DNA-delivery agent that gets engulfed by cells so the DNA can potentially become integrated into the mouse cell chromosomes," Freimuth said. "To find the cells that picked up the foreign gene(s), we add the antibiotic to the cell cultures. Cells that failed to take up and express the antibiotic-resistance gene died, whereas those that acquired antibiotic resistance survived and grew into colonies."
The scientists expanded about 50 of those colonies into individual cultures and then tested them to determine how many had also picked up the human ACE2 gene and produced the human receptor protein.
Detecting protein production
"About 70% of the antibiotic-resistant colonies expressed the human ACE2 protein on the cell surface," Freimuth said. "Further analysis showed that these colonies contained, on average, 28 copies of the human ACE2 gene."

Importantly, the mouse cells held onto the "foreign" ACE2 gene copies and kept making the human ACE2 protein encoded by these genes for at least 90 cell generations.
The level of human ACE2 protein produced by the cells was generally proportional to the number of ACE2 gene copies integrated into the mouse genome. Several of the mouse cell clones produced about 50 times more ACE2 than is normally present on mouse cells.
The scientists used a variety of methods to test whether the mouse-made human ACE2 proteins were functional. These included demonstrating that a "pseudovirus" containing the COVID spike protein—that is, a non-pathogenic stand-in for SARS-CoV-2—could bind to the receptors and infect the cells.
"These infectivity assays showed that the human ACE2 protein expressed on these mouse cells is fully functional," Freimuth said.
Uses and implications
Meanwhile, Oleg Gang and Feiyue Teng, study co-authors from CFN, explored various ways to create extracellular nano-vesicles enriched with decoy human ACE2 for the potential treatment of COVID-19. They are also investigating the placement of ACE2 proteins onto nanoparticles for potential applications in infection treatment or rapid virus detection.
"The challenge posed by ACE2-based nano-vesicles lies in enhancing their neutralization effect against SARS-CoV-2. We are also looking for ways to enhance and leverage the binding sensitivity and specificity of ACE2-coupled nanoparticles to make them useful for virus diagnostics. Both approaches would require future optimization efforts," said Teng, a research associate at CFN who worked extensively on both the biological aspects of this study and the potential nanoscience-based applications.
"We are excited to combine advances in nanomaterial fabrication with biomolecular approaches for developing new therapeutic and sensing strategies," said Gang, who holds a joint appointment at Columbia University. "This study allowed us to overcome some methodological problems since nanomaterials and biosystems required quite different characterization approaches. What we have learned here is important for our next steps in enhancing nanoparticle-based biosensing."
In addition to enabling possible applications of recombinant ACE2 protein, the work also demonstrates a novel approach for producing a wide range of complex proteins. Examples include the vast array of cell-surface receptors that mediate countless biological and disease processes, as well as industrially important proteins such as monoclonal antibodies and enzymes.
"Our method of using intact genes along with mouse cells that can be adapted to grow in huge suspension cultures—much like the liquid broth cultures used to grow bacteria—could advance the large-scale production of these and other important proteins," Freimuth said.
More information: Feiyue Teng et al, Overexpression of human ACE2 protein in mouse fibroblasts stably transfected with the intact ACE2 gene, Virology (2024). DOI: 10.1016/j.virol.2024.109988
Journal information: Virology
Provided by Brookhaven National Laboratory