This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study reveals mechanisms behind how growing cells maintain their mojo by scaling up biosynthesis
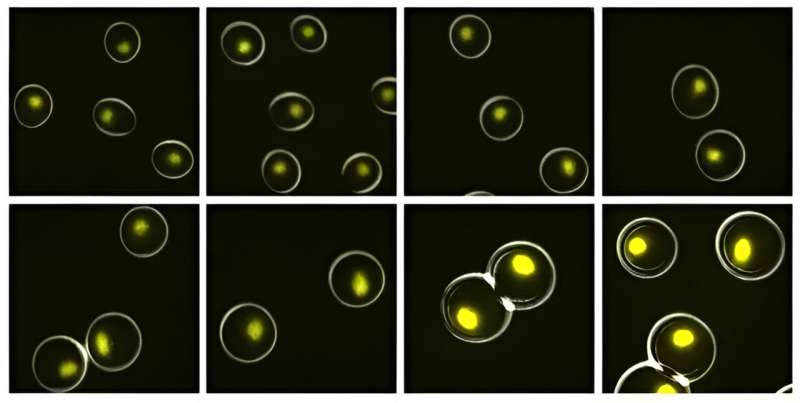
Similar to the way expanding companies increase their work forces, cells must boost their production of internal biomolecules when they grow in size in order to stay healthy. In the 1970s, biologists showed that this scaled-up biosynthesis hinges on faster rates of transcription—the process where genetic blueprints in DNA are copied over to RNA molecules. Yet in the half-century since, the mechanism behind the expedited transcription rates has remained unclear.
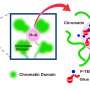
Now Stanford researchers report in a new study published in Cell that they have cracked the case. The researchers discovered that growing cells can crank out the RNA they need thanks to increasing availability of a critical enzyme, called RNA polymerase II (RNAPII), that escalates with cell size. The enzyme binds to DNA to make messenger RNA (mRNA)—an essential molecule that delivers instructions to a cell's protein-making factories.
In this way, cells of varying sizes can proportionally maintain their biomolecules at near-constant concentrations and continue functioning efficiently as they grow. Besides shedding new light on fundamental cellular biology, the findings are significant because breakdowns in biosynthetic scaling likely play a major role in the deterioration of cells, leading to diseases and aging.
"In this study, we have answered the long-standing question: How does transcription increase with cell size?" said Matthew Swaffer, lead author of the study. "As cells grow and get larger, they need to increase the synthesis of everything within them, and now we have a much better understanding of the processes and mechanisms involved."
Swaffer conducted the work as a postdoctoral researcher in the lab of Jan Skotheim, a professor of biology in Stanford's School of Humanities and Sciences. Swaffer is now a group leader at the Wellcome Center for Cell Biology at the University of Edinburgh.
In their study, the Stanford researchers also discovered a supplemental mechanism that kicks in when cells get too big and there simply isn't enough RNAPII to go around. Somehow, the stability of mRNA molecules increases, enabling a cell to, at least temporarily, keep its overall biology in balance and thus extend its life.
"Transcription is almost proportional to cell size because of the increasing number of RNAPII molecules in larger cells driving an increase in transcription, but this is not enough for the largest cells," said Skotheim, the senior author of the study. "We identified a new feedback mechanism that stabilizes mRNA when its concentration starts to go down. Working together, these two mechanisms are able to maintain mRNA concentration over a large range of cell sizes."
Tracking down a pivotal biomolecule
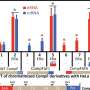
To arrive at these insights, Swaffer first looked at the list of biomolecules known to be essential for transcription. Swaffer then devised an experiment where yeast cells were only able to use half the usual amount of each of the critical biomolecules in question to drive transcription. Swaffer and colleagues then measured any effects in transcription related to these altered biochemical concentrations.
Interestingly, transcription was not affected in the slightest with any biomolecule Swaffer tested except RNAPII. "A big surprise in this study was that we could remove half of the typical amount of these transcriptional factors and have basically no change in transcription," Skotheim said. "The only thing that caused transcription to drop was removing half the amount of RNAPII."
Based on this straightforward finding, the Stanford researchers constructed a dynamic equilibrium model for how cell size and RNAPII-powered transcription rates stay synced. When transcription alone can no longer adequately scale in cells, the additional mechanism of enhanced mRNA persistence switches on. Swaffer is currently investigating the biology behind this newly discovered stability of mRNA when transcription alone can no longer adequately scale in cells.
Connections to aging and disease
Overall, the findings offer a new window into the growth, maturation, and eventual decline of cells.
In this vein, a growing number of studies have started drawing connections between declines in biosynthesis scaling and cellular function. For example, in very large cells where this scaling is failing, growth rates decrease sharply, cell-to-cell communication breaks down, and gene activation malfunctions.
Cells in this state are experiencing what is known as cellular senescence. Such cells cannot multiply anymore, as cells normally do, but they don't die off, either. Instead, senescent cells contribute to tissue and organ dysfunction, inflammation, and other health issues.
Furthermore, in looking at cell size in reverse, among the smallest cells in the body are stem cells—the cells from which all other cell types emerge. As organisms age, though, stem cells enlarge in size and lose some of their ability to make new cells.
"As cells get bigger and bigger and the transcription scaling and mRNA stability mechanisms that we describe in this study become insufficient, then you observe that concentrations of mRNA decline and the cells start not working as well," Skotheim said. "The hypothesis we have now is that this breakdown in mRNA homeostasis is one of the first steps toward cellular senescence, and we plan to test this hypothesis in future work."
More information: Matthew P. Swaffer et al, RNA polymerase II dynamics and mRNA stability feedback scale mRNA amounts with cell size, Cell (2023). DOI: 10.1016/j.cell.2023.10.012
Journal information: Cell
Provided by Stanford University