April 3, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
From yeast to mice, from mice to man: Senescent cells get noisier with age
Getting old seems completely avoidable in youth but becomes less and less so as we age. Many of the obstacles of advanced aging are well understood, including declining eyesight, hearing loss, back, neck and arthritic joint pains, shortness of breath, diabetes, Alzheimer's disease, dementia, cancer and stroke, just to name a few. What is less understood are the cellular-level molecular mechanisms responsible for our overall decline.
Researcher Payel Sen of the University of Pennsylvania and colleagues pursued an investigation into the chromatin-mediated loss of transcriptional fidelity during human cellular senescence. Their findings are described in an article titled "Spurious intragenic transcription is a feature of mammalian cellular senescence and tissue aging," published in the journal Nature Aging. Nikita Isima and Jesús Gil published a News & Views piece on this scholarship in the same journal issue.
Senescence is the step cells take when they determine it is best not to replicate, inhibiting the proliferation of abnormal cells by altering chromosomal configurations. Essentially as we age, our dividing cells accumulate mutations, little changes from one generation of cells to the next. Enough of these and the cell is at risk of being unsustainable or becoming a tumor cell. In this sense, senescence plays an essential role in limiting tumor progression.
Senescence is also a response to damage, allowing for the suppression of damaged or poorly repaired cells or damage of the telomeres. Accelerated accumulation of senescent cells with age is associated with various forms of disease—osteoarthritis, lung disease, Alzheimer's, dementia and cancer, to name a few.
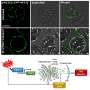
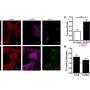
The study by Sen and colleagues found that during aging and senescence, cryptic transcription (proteins not normally produced) are suddenly created inside cells and that this is related to changes in the chromatin landscape. The study proposes that cryptic transcription is spurious and not created for a specific purpose. The noisy cryptic transcriptome then competes indirectly with coherent transcriptional networks by expending cells' energy on their production. Researchers saw no evidence of cryptic transcription being sensed as damage by the cell, nor do they think it is causative of senescence but simply a result of changes brought on by senescence.
The study, along with an experiment in mouse livers, builds on previous observations of cryptic transcription and indicates that this senescence mechanism is conserved from yeast to mice and humans. The study also finds, similar to previous reports, that cryptic transcription tissue location is sex-dependent, as males and females will manifest spurious proteins in different cell types.
More information: Payel Sen, Spurious intragenic transcription is a feature of mammalian cellular senescence and tissue aging, Nature Aging (2023). DOI: 10.1038/s43587-023-00384-3. www.nature.com/articles/s43587-023-00384-3
Isima, N., Gil, J. Spurious transcription may be a hallmark of aging. Nature Aging (2023). DOI: 10.1038/s43587-023-00398-x. www.nature.com/articles/s43587-023-00398-x
Journal information: Nature Aging
© 2023 Science X Network