This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Meet pAblo·pCasso: A new leap in CRISPR technologies for next-gen genome engineering
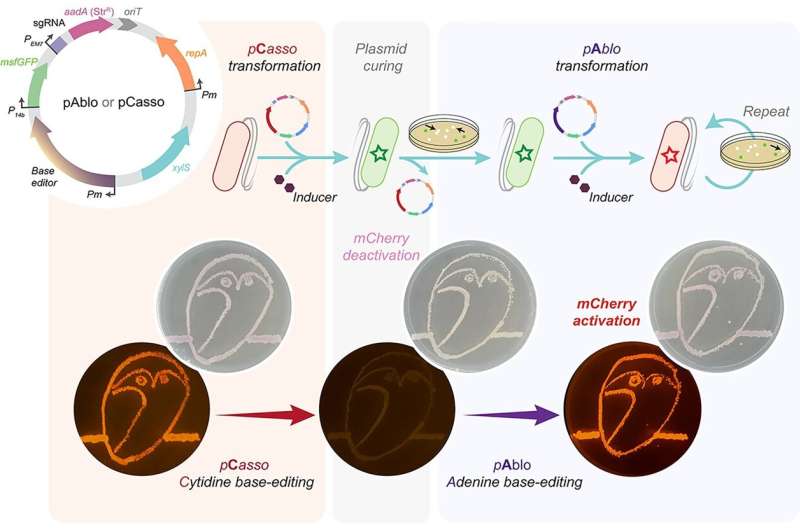
A new CRISPR-Cas toolkit, dubbed "pAblo·pCasso," is set to transform the landscape of bacterial genome editing, offering unprecedented precision and flexibility in genetic engineering. The new technology, developed by researchers at The Novo Nordisk Foundation Center for Biosustainability (DTU Biosustain), expands the range of genome sites available for base-editing and dramatically accelerates the development of bacteria for a wide range of bioproduction applications.
pAblo·pCasso sets a new standard in CRISPR-Cas technologies. A key innovation is to enable precise and reversible DNA edits within Gram-negative bacteria, a feat not achievable with previous CRISPR systems. The toolkit utilizes specialized fusion enzymes, modified Cas9 coupled with editor modules CBE or ABE, which act like molecular pencils to alter specific DNA nucleotides, thus accurately controlling gene function.
The development of pAblo·pCasso involved overcoming significant challenges. Traditional CRISPR-Cas systems were limited by their need for specific DNA sequences (PAM sequences) near the target site and were less effective in making precise, single-nucleotide changes. pAblo·pCasso transcends these limitations by incorporating advanced Cas-fusion variants that do not require specific PAM sequences, thereby expanding the range of possible genomic editing sites.
Furthermore, its reversible editing capability, achieved through specialized enzymes, allows for temporary modifications, essential for dynamic and controlled gene studies.
Breaking the barriers of traditional CRISPR technology
This technology dramatically enhances the capabilities of researchers and industries in engineering bacterial cell factories. By facilitating rapid and precise genetic modifications, pAblo·pCasso accelerates the development of bacteria for a wide array of bioproduction applications, from pharmaceuticals to biofuels, aligning with sustainable production goals.
Professor Pablo I. Nikel from DTU Biosustain says, "With pAblo·pCasso, we've broken the barriers of traditional CRISPR technology. This toolkit opens up new possibilities for bacterial engineering, bringing us closer to program efficient and sustainable bioproduction with engineered bacteria."
Postdocs Dr. Ekaterina Kozaeva and Manuel Nieto-Domínguez add, "A major novelty of this approach lies in its ability to access and engineer previously unavailable sites for genome editing while leaving no traces afterward. We can now create bacterial cell factories in days instead of months. Tools like the pAblo·pCasso plasmids redefine our approach to genetic manipulation of bacteria, especially atypical ones that are usually more difficult to engineer."
The study is published in the journal Nucleic Acids Research.
More information: Ekaterina Kozaeva et al, The pAblo·pCasso self-curing vector toolset for unconstrained cytidine and adenine base-editing in Gram-negative bacteria, Nucleic Acids Research (2024). DOI: 10.1093/nar/gkad1236
Journal information: Nucleic Acids Research
Provided by Danmarks Tekniske Universitet The Novo Nordisk Foundation Center for Biosustainability