This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Molecular 'super-glue' shows promise of cancer drug discovery platform
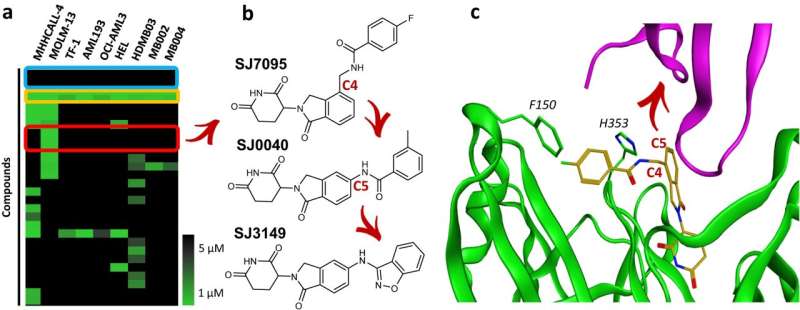
St. Jude Children's Research Hospital scientists have published their work on SJ3149, a compound with broad activity against many cancer types, particularly acute myeloid leukemia (AML). SJ3149 sticks to the cancer-related protein casein kinase 1 alpha (CK1α), leading to its destruction.
The work is published in the journal Nature Communications.
"We have made a molecular super-glue," said senior co-corresponding author Zoran Rankovic, Ph.D., St. Jude Department of Chemical Biology and Therapeutics. "SJ3149 is the first-in-class potent and selective CK1α degrader, showing efficacy in both in vitro and in vivo cancer models."
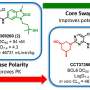
Molecular glues act by hijacking the cell's natural protein recycling mechanism. The molecular glue recruits the targeted protein to an enzyme that marks it for destruction through a process called proteasomal degradation. For many cancer-related proteins that cannot be targeted well by conventional small molecule inhibitors, molecular glues may be a viable therapeutic alternative. This led the scientists at St. Jude to develop a large proprietary library of molecular glues and screen it against a range of cancer cell lines, finding an initial hit.
Once the researchers optimized the identified hit, the resulting SJ3149 molecule showed higher potency and less off-target effects than similar compounds. SJ3149 displayed auspicious broad anti-cancer activity even for a molecular glue, hence the moniker "super-glue." The compound also appears to have a similar profile to a class of approved cancer drugs, murine double minute 2 (MDM2) inhibitors, further indicating it may have clinical utility.
Platform for molecular glue discovery
Molecular glues are a promising well from which to find new therapies because they can target previously undruggable proteins. However, finding and adapting these molecules to clinical use has been challenging. Identifying and refining such a molecule provides proof of concept that the St. Jude approach can accelerate this discovery process.
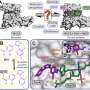
"Our work provides a blueprint to conduct similar studies for other targets," said co-corresponding author Marcus Fischer, Ph.D., St. Jude Department of Chemical Biology and Therapeutics. The researchers discovered the compound, altered it through rational design and tested its efficacy. To understand how the compound worked so well, Fischer's group crystallized the large complex of the targeted protein and SJ3149 bound to the cell's machinery responsible for tagging proteins for degradation, a protein ubiquitin ligase apparatus.
"We could see that the beauty of this compound is that it directly interacts with CK1α," Fischer explained. "SJ3149 reaches over and directly connects CK1α to the enzyme that marks it for the cellular degradation machinery, providing a rationale for the compound's high degradation efficacy."
Understanding how such compounds work at the atomic level may pave the way for the rational design of molecular glues.
"This is a perfect example of taking chemical matter and making structural and mechanistic insights to understand efficacy and cellular activity," said co-corresponding author Jeffery Klco, MD, Ph.D., St. Jude Department of Pathology, a physician-scientist with a focus on AML. "At this stage, it is still just a lead compound, but this may develop into another potential option for treating different pediatric cancers, which is exciting."
Creating the compound was a major collaborative effort. The work entailed designing, synthesizing and screening the molecular glue library, structure-guided medicinal chemistry optimization, and testing in patient-derived cancer cells. It was only possible through the combined expertise of Rankovic, Fischer and Klco's labs, in concert with internal and external collaborators. The approach can now be used as the basis for further discovery.
"Chemical biology has entered a new paradigm with molecular glues," Rankovic said. "With this study, we have now established a pipeline to identify novel promising molecular glues for cancer treatments."
More information: Gisele Nishiguchi et al, Selective CK1α degraders exert antiproliferative activity against a broad range of human cancer cell lines, Nature Communications (2024). DOI: 10.1038/s41467-024-44698-1
Journal information: Nature Communications
Provided by St. Jude Children's Research Hospital