January 24, 2024 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New method for incorporating structurally unusual amino acids into proteins
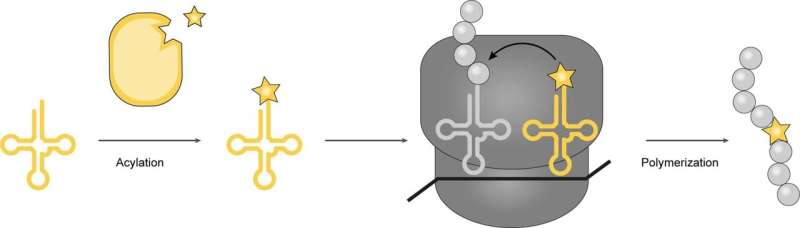
A team of biochemists at the Medical Research Council Laboratory of Molecular Biology at Cambridge has developed a new method to incorporate structurally unusual amino acids into proteins by using bacteria. The method is described in the journal Nature.
Prior research has shown that DNA codes for just 20 amino acids, the building blocks for making all the proteins used by living creatures. These are known as alpha amino acids. Prior research has also suggested that beneficial compounds could be created with a method to create proteins using beta amino acids. Researchers have suggested that applications could include the development of new kinds of medicines and possibly novel catalysts for manufacturing processes.
Such proteins have been engineered via syntheses in the lab. but scientists would prefer a more natural approach, which would be both cheaper and more efficient. This means that a technique is required to get a living cell to generate a desired protein using a beta amino acid.
Prior research suggests that would involve first getting a cell to carry an amino acid to a ribosome, and then getting the ribosome to integrate it into the protein. Some in the field have likened the process to loading railway cars and then linking them together.
In this new effort, the researchers accomplished this feat by changing the way a bacteria's protein-making machine works so that it could make use of new kinds of amino acids to build desired proteins.
The work involved creating mutated genes for enzymes that in turn load a desired amino acid onto snippets of transfer RNA, where they travel to the ribosome and bind to sequences of messenger RNA. Following that, the ribosome pulls off amino acids and links them together in chains used in the new protein. Using this approach, the research team was able to incorporate four exotic amino acids into a protein.
More information: Daniel L. Dunkelmann et al, Adding α,α-disubstituted and β-linked monomers to the genetic code of an organism, Nature (2024). DOI: 10.1038/s41586-023-06897-6
Journal information: Nature
© 2024 Science X Network