This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A surprisingly simple expression for enzyme activity could help guide biotechnologists
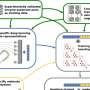
![Relationship between thermodynamic landscapes and enzymatic activity. Three thermodynamic landscapes are shown in A. Their corresponding Michaelis-Menten plots are shown in B. The Km values are indicated as vertical dashed lines in B. Increasing the driving force of the first step increases the activity at low substrate concentrations but lowers the activity at high substrate concentrations. Therefore, the thermodynamic landscape of an optimum enzyme depends on the substrate concentration ([S]). Credit: Nature Communications (2023). DOI: 10.1038/s41467-023-40471-y A surprisingly simple expression for enzyme activity could help guide biotechnologists](https://scx1.b-cdn.net/csz/news/800a/2023/a-surprisingly-simple-2.jpg)
A surprising relationship that governs the activity of enzymes—the molecules that catalyze almost all the chemical reactions of life—has been uncovered by three RIKEN scientists. This finding could help researchers to select and design the best enzymes for use in biotechnology applications. The study is published in the journal Nature Communications.
All forms of life depend on enzymes—without them most biochemical reactions would proceed too slowly to sustain the processes of life. Enzymes function by binding to a compound known as a substrate.
The activity of enzymes—a measure of how much they speed up a reaction—is commonly described mathematically by considering three rates: the rate at which the substrate molecules bind to the enzyme; the rate at which that binding can be reversed; and the rate at which the substrate is converted into the reaction's product.
Each of these three steps is characterized by a numerical rate constant. When these constants are combined in an equation, a value called Km emerges. This reflects the affinity of the enzyme for its substrate, with lower Km values indicating higher affinity.
The RIKEN trio's mathematical analysis revealed a surprisingly simple relationship between an enzyme's Km value and the conditions in which it will be the most active enzyme among enzymes that catalyze the same reaction.
"Theory predicts that the best enzyme is one that has a Km equal to the substrate concentration," says Hideshi Ooka of the RIKEN Center for Sustainable Resource Science. "We started this research knowing that we would obtain some kind of formula for maximum activity, but we never expected it to be so concise. For me, the simplicity felt beautiful in a mathematical sense."
This insight led the team to explore existing data for the relationship between Km and substrate concentrations in nature. The results supported their hypothesis: A survey of more than 1,000 enzymes revealed that many operate in surroundings in which the concentration of their substrate was equal to, or very close to, their individual Km values.
"This suggests that one direction of biological evolution was to ensure that the Km values of enzymes are close to the substrate concentrations in their natural environment," says Ooka.
While offering an important new understanding of enzyme evolution, this insight will also help researchers to modify or design enzymes for use in biotechnology.
"One key takeaway message is that Km should not be too small, contradicting a previous assumption that a small Km is always better," Ooka says. "Instead, choosing or designing enzymes with Km values equal to the substrate concentrations they will have to work with will be the best strategy."
More information: Hideshi Ooka et al, Thermodynamic principle to enhance enzymatic activity using the substrate affinity, Nature Communications (2023). DOI: 10.1038/s41467-023-40471-y
Journal information: Nature Communications
Provided by RIKEN