December 5, 2023 dialog
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
written by researcher(s)
proofread
How a pathogenic bacterium uses molecular mimicry to compromise a cell's protein building factory
The central dogma of molecular biology postulates that the information packets encoded within the molecules of deoxyribonucleic acid (DNA) are first transcribed into molecules of messenger ribonucleic acids (mRNAs), and then subsequently translated/decoded to generate molecules called proteins.
Proteins are essential biomolecules that are composed of multiple smaller subunits called amino acids. These amino acids are stitched together via peptide bonds and contribute to the shape, size and charge distribution that the protein, as a sum of its amino acid parts, eventually exhibits.
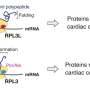
For cells to make proteins, they need to decode the language of the mRNA (nucleic acid) and translate that into the language of proteins (amino acid). This process is described in molecular biology textbooks interchangeably as mRNA translation or protein synthesis.
Inside cells, translation is carried out by a molecular decoding center called the ribosome. The ribosome itself consists of dozens of proteins and RNAs. In addition, many other regulatory factors associate with the ribosome to help make the translation process fast, accurate and tunable. In fact, if you count the number of proteins involved in this process, it is more than 100—yes, it takes more than 100 proteins to make one! So the process of translation is extremely energy and resource-consuming, reflecting its huge importance to the cell.
During translation, a key molecule that facilitates decoding is called a tRNA (transfer RNA). The tRNA is the "translator" that knows both the language of the nucleic acid and the language of the proteins. tRNAs bring an amino acid (protein building block) to the matching mRNA sequence (called the codon), while the ribosome moves along the length of the mRNA and simultaneously stitches the amino acids together. Thus, the ribosome-tRNA complex translates one language to another, executing the key step in protein synthesis.
As it is with any process, cells try their very best to maintain the efficiency and precision of protein synthesis. Indeed, measurements in many different cells have determined that proteins are generally synthesized with high accuracy at an average speed of ~6 amino acids per second.
Pathogens target protein synthesis.
Numerous pathogens including viruses, bacteria and fungi have evolved mechanisms that directly target the protein synthesis machinery in the cells they infect. This allows for the pathogens to propagate, to subvert host cell defenses and ultimately to regulate host cell lysis so that the progeny can be released for further rounds of infection.
In these infected cells, the elongation of amino-acid chains that constitute proteins does not progress at a uniform rate, resulting in a pile-up of ribosomes at specific positions on the mRNAs being translated and a drop in the rates of host cell protein synthesis. For an analogy, think of rush hour traffic on a freeway where the cars are the ribosomes and the asphalt road is the length of an mRNA. Due to an untoward incident, cars begin to pile up on the freeway and the rate of traffic slows down.
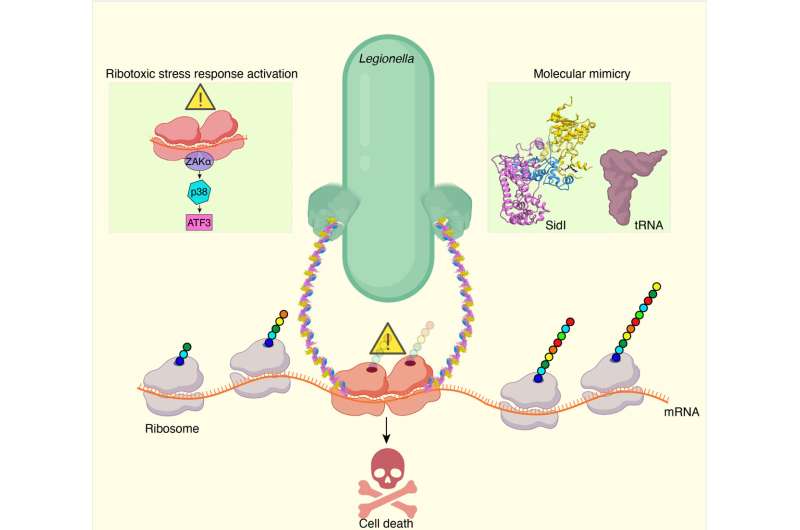
Past evidence has indicated that a pathogenic bacterium called Legionella pneumophila causes a similar reaction in the cells it infects—a reduction in host cell protein synthesis rates and a pausing of ribosomes on mRNAs, that is, traffic jams on the "protein synthesis freeway." Legionella pneumophila is an intracellular bacterium (it infects and resides within the cells where it replicates) and is the causative organism of Legionnaires' disease, a debilitating atypical form of pneumonia.
We asked how and why Legionella pneumophila targets the step of translation elongation. And we set out to answer these questions in a recent study now published in Nature Cell Biology.
Our path to discovery is filled with surprises
Legionella secretes a few toxins into an infected cell. We therefore first measured if these toxins might be equipotent in their ability to inhibit protein synthesis. Here was our first surprise: While six of the toxins we tested had similar effects, one Legionella toxin stood out from the rest of the pack, and this was called SidI (pronounced "Sid-eye"). We discovered that minute quantities of SidI robustly inhibited protein synthesis. Our measurements indicated that SidI's potency is comparable to the potency of ricin, one of the most potent toxins in nature.
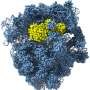
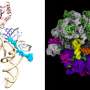
Our second surprise was realized when we solved the structure of this potent inhibitor by cryo-electron microscopy, a technique that preserves the three-dimensional architecture of a biomolecule in solution by embedding it into an environment of vitreous ice (approximately -320°F). Our structure revealed a fascinating molecular mimicry.
While one-half of SidI has an architectural fold present in enzymes called glycosyl transferases, the other half resembles the shape and size of a molecule of tRNA. Remember that tRNAs bring individual amino acids to the ribosomes to help decode mRNAs. SidI pretends to be a tRNA and tricks the ribosome into accepting it, but instead of bringing an amino acid to the ribosome, it stops the ribosome from translating.
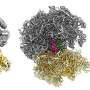
We performed a battery of experiments to determine that SidI directly targets host cell ribosomes and modifies them. This results in a condition wherein the modified ribosome on an mRNA moves much more slowly than the ribosome trailing it, causing ribosome collisions. In sticking with the freeway analogy, this is like car collisions occurring when the car in front of you has problems and slows down suddenly.
To our knowledge, this coupling of form (tRNA mimicry) and function (enzymatic activity) makes SidI a unique and unprecedented molecule in nature.
"What, then, are [the] consequences of such aberrant collided ribosomes in infected cells?" we asked.
In trying to address this question, we got our third surprise. We discovered that these collided ribosomes are sensed by host cells to activate a ribotoxic stress response pathway that culminates in the accumulation of a master regulator of gene expression called activating transcription factor 3 (ATF3).
Remarkably, even while most protein synthesis is inhibited, ATF3 protein escapes this mRNA translation block and is induced at high levels. ATF3 enters the nucleus of cells and orchestrates a program that regulates cell lysis. We think this mechanism might be important for the escape of replicated bacteria into the extracellular milieu, facilitating further rounds of infection.
Perspectives
Toxins from pathogenic microorganisms have long been used as precise molecular instruments to interrogate fundamental processes that occur within cells. SidI now joins this arsenal of nature's tools. The fundamental insights gained from our studies shed new light on the mechanisms by which pathogens employ molecular mimicry to hijack processes that are critical for monitoring optimal host cell function.
Interestingly, through our elucidation of SidI's molecular mechanism, we have in turn unearthed critical signaling nodes of the stress response pathway that is activated downstream of colliding ribosome stress. As our mentor, Shaeri Mukherjee, a professor at the University of California San Francisco always says—"Bacteria are the best cell biologists!"
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about ScienceX Dialog and how to participate.
More information: Advait Subramanian et al, A Legionella toxin exhibits tRNA mimicry and glycosyl transferase activity to target the translation machinery and trigger a ribotoxic stress response, Nature Cell Biology (2023). DOI: 10.1038/s41556-023-01248-z
Journal information: Nature Cell Biology
Advait Subramanian is a cell biologist working at Altos Labs Inc., Redwood City, California. Previously, Subramanian was a postdoctoral fellow mentored by Shaeri Mukherjee and Peter Walter at the University of California, San Francisco where the work described in this article was carried out. Email for correspondence: asubramanian@altoslabs.com
Lan Wang is a structural biologist and Assistant Professor working at The Hong Kong University of Science and Technology, Hong Kong. Previously, Lan was a postdoctoral fellow mentored by Peter Walter at the University of California, San Francisco. Email for correspondence: lanwang@ust.hk