This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Developing nanocatalysts to overcome limitations of water electrolysis technology
Green hydrogen can be produced through water electrolysis technology, which uses renewable energy to split water into hydrogen and oxygen without emitting carbon dioxide. However, the production cost of green hydrogen is currently around $5 per kilogram, which is two to three times higher than gray hydrogen obtained from natural gas.
For the practical use of green hydrogen, innovation in water electrolysis technology is required for the realization of hydrogen economy, especially for Korea where the utilization of renewable energy is limited owing to geographical reasons.
Dr. Kyung Joong Yoon's research team at the Energy Materials Research Center of the Korea Institute of Science and Technology (KIST) has developed a nanocatalyst for high-temperature water electrolysis that can retain a high current density of more than 1A/cm2 for a long period of time at temperatures above 600°. The work is published in the Chemical Engineering Journal.
While the degradation mechanisms of nanomaterials at high temperatures have been elusive thus far, the team identified the fundamental reasons of abnormal behavior of nanomateirals and successfully resolved issues, eventually improving performance and stability in realistic water electrolysis cells.
The electrolysis technology can be classified into low- and high-temperature electrolysis. While low-temperature electrolysis operating at temperatures below 100° Celsius has long been developed and is technologically more mature, high-temperature electrolysis operating above 600° Celsius offers higher efficiency and is considered as a next-generation technology with a strong potential for further cost-down.
However, its commercialization has been hindered by the lack of thermal stability and insufficient lifetime owing to high-temperature degradation, such as corrosion and structural deformation. In particular, nanocatalysts, which are widely used to improve the performance of low-temperature water electrolyzers, quickly deteriorate at high operating temperatures, making it difficult to effectively use them for high-temperature water electrolysis.
To overcome this limitation, the team developed a new nanocatalyst synthetic technique that suppresses the formation of harmful compounds causing high temperature degradation.
By systematically analyzing the nanoscale phenomena using transmission electron microscopy, the researchers identified specific substances causing severe structural alterations, such as strontium carbonate and cobalt oxide and successfully removed them to achieve highly stable nanocatalysts, in terms of chemical and physical properties.
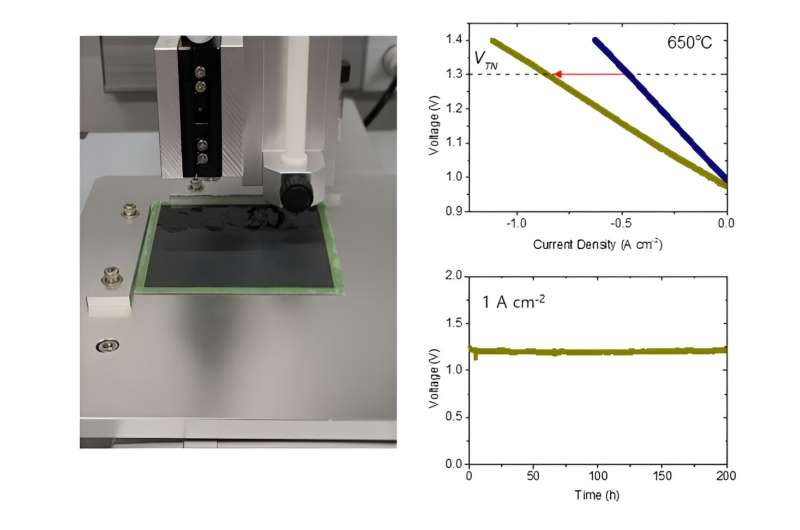
When the team applied the nanocatalyst to a high-temperature water electrolysis cell, it more than doubled hydrogen production rate and operated for more than 400 hours at 650° without degradation. This technique was also successfully applied to a practical large-area water electrolysis cell, confirming its strong potential for scale-up and commercial use.
"Our newly-developed nanomaterials achieved both high performance and stability for high-temperature water electrolysis technology, and it can contribute to lower the production cost of green hydrogen, making it economically competitive with gray hydrogen in the future," said Dr. Kyungjoong Yoon of KIST.
"For commercialization, we plan to develop automated processing techniques for mass production in cooperation with industry cell manufacturers."
More information: Mi Young Park et al, In situ synthesis of extremely small, thermally stable perovskite nanocatalysts for high-temperature electrochemical energy devices, Chemical Engineering Journal (2023). DOI: 10.1016/j.cej.2023.146924
Journal information: Chemical Engineering Journal