This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
MAVS found to antagonize human stem cell senescence as a mitochondrial stabilizer
A recent study led by Professor Weiqi Zhang, affiliated with the Beijing Institute of Genomics, Chinese Academy of Sciences, and the China National Center for Bioinformation, in collaboration with Professors Guang-Hui Liu and Jing Qu from the Institute of Zoology, Chinese Academy of Sciences, has successfully illuminated the intricate molecular mechanism through which the protein MAVS, associated with the innate immune response, governs the senescence of human stem cells.
Their research has unveiled an unconventional role of MAVS that extends beyond its established immune regulatory functions, as it assumes a pivotal role in upholding both structural and functional equilibrium within mitochondria, thereby influencing the senescence of human stem cells. This discovery has been detailed in their publication in the journal Research, titled "MAVS antagonizes human stem cell senescence as a mitochondrial stabilizer."
MAVS is an antiviral signaling protein located on the mitochondrial membrane, playing a pivotal role in defending RNA virus infection. Currently, most studies have focused on the classical innate immune regulatory function of MAVS in response to viral infection. However, as a key switch molecule in the innate immune pathway, does MAVS accelerate or delay human stem cell senescence?
Moreover, as a protein localized in the mitochondria, is MAVS linked to mitochondrial homeostasis, which in turn regulates stem cell function? Answering these questions will deepen our understanding of the innate immune pathway, mitochondrial biology, and aging biology, revealing the profound connections between innate immunity, mitochondrial homeostasis, and stem cell senescence.
It's worth noting that mitochondrial dysfunction is a hallmark feature of cellular senescence and organ aging. Growing evidence demonstrates that mitochondrial dysfunction is closely related to stem cell exhaustion and cellular senescence. Therefore, identifying key stabilizers that maintain mitochondrial structure and function is significant in alleviating cellular senescence and organismal aging.
As an important protein located on the mitochondrial membrane, the mitochondrial interaction protein profile of MAVS in human stem cells has not been described, and whether it participates in mitochondrial homeostasis regulation has not been revealed. The role and mechanism of MAVS in the regulation of human stem cell senescence remain unclear.
To investigate MAVS's function across various human stem cell types, the researchers first employed CRISPR/Cas9-mediated gene editing coupled with stem cell-directed differentiation techniques to generate MAVS gene-specific knockout human embryonic stem cells, human neural stem cells and human mesenchymal stem cells.
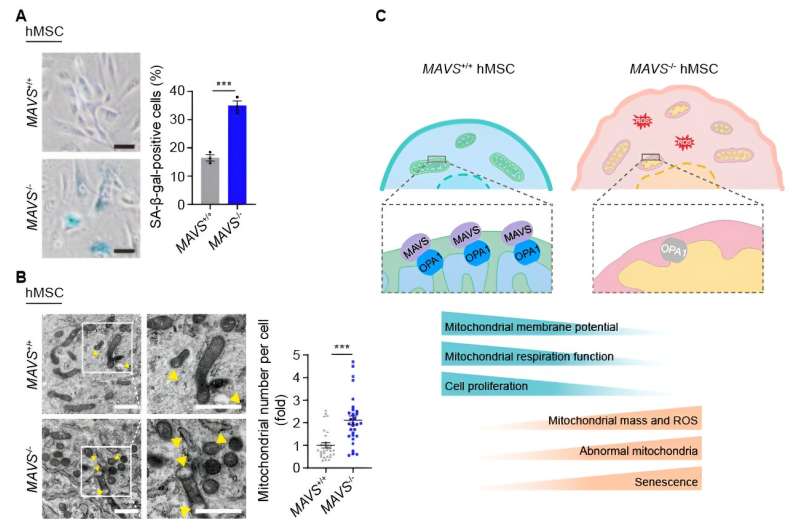
Although the deletion of MAVS did not affect the self-renewal and differentiation of human embryonic stem cells and human neural stem cells, MAVS-knockout human mesenchymal stem cells exhibit a series of senescence-associated defects. This suggests that MAVS likely exerts a cell-type-specific role in maintaining human stem cell function, and human mesenchymal stem cells appear to be more vulnerable to the absence of MAVS.
Considering the regulatory function of MAVS in innate immune response, the researchers found that the canonical downstream signaling responses of MAVS, such as NF-κB, IRF3, and IFNα, were downregulated in MAVS-knockout human mesenchymal stem cells, and senescence-associated secretory phenotype was also decreased.
This indicates that the absence of MAVS at least partially inhibits downstream immune inflammation. However, these observations are seemingly contradictory to the prevailing notion that increased SASP is not only the hallmark but also the key driver of many pathological changes of aging. This prompts the question of whether MAVS might regulate human stem cell senescence through a distinct pathway.
Interestingly, the researchers noted heightened mitochondrial mass and anomalous mitochondria proportion in MAVS-knockout human mesenchymal stem cells, implying an alteration in mitochondrial structure. Additionally, a reduction in mitochondrial membrane potential, elevated mitochondrial ROS levels, and a decrease in mitochondrial oxidative respiration rate were observed in these MAVS-knockout human mesenchymal stem cells, collectively indicating that the absence of MAVS precipitates mitochondrial dysfunction.
Furthermore, the researchers analyzed the molecular mechanism of MAVS-mediated human stem cell senescence regulation. They found the interaction between MAVS and OPA1, a protein associated with mitochondrial fusion. Moreover, the absence of MAVS led to the downregulation of the OPA1 protein level. Similarly, in replicative senescent human mesenchymal stem cells, the researchers also observed the downregulation of both MAVS and OPA1 protein levels.
To verify the function of MAVS-OPA1 axis in regulating mitochondrial homeostasis and cellular senescence, the researchers found that OPA1 knockdown could mimic the deficiency caused by MAVS depletion on mitochondrial structure and function, and further aggravation of cellular senescence was observed due to OPA1 knockdown.
In addition, the reintroduction of OPA1 or MAVS itself in MAVS-knockout human mesenchymal stem cells was found to restore mitochondrial structural and functional homeostasis and ultimately alleviate cellular senescence. In aggregate, this study reveals that MAVS maintains mitochondrial homeostasis and regulates cellular senescence through stabilizing OPA1.
This study has illuminated a non-canonical geroprotective function of the conventional innate immune pathway-associated protein, MAVS, in sustaining mitochondrial equilibrium and governing the senescence of human stem cells. The research not only provides novel perspectives on the biological role of MAVS but also furnishes valuable indications for mitigating stem cell senescence and pursuing therapies for age-related ailments.
In the future, further investigations are needed to explore how MAVS finely regulates mitochondria at different levels and how it coordinates its roles in antiviral immune response and mitochondrial homeostasis during the aging process.
More information: Cui Wang et al, MAVS Antagonizes Human Stem Cell Senescence as a Mitochondrial Stabilizer, Research (2023). DOI: 10.34133/research.0192
Journal information: Research
Provided by Research