A host protein phosphatase restricts innate immune signaling
The adaptor proteins STING and MAVS are components of critical pathogen-sensing pathways that induce innate immunity. Phosphorylation of either adaptor results in activation of the type I interferon pathway and excessive activation of the system is sometimes associated with fatal inflammatory diseases.
Thus, the activity of the system, and in particular, the activities of the innate immune adaptors, must be precisely regulated to ensure a proper and balanced innate immune homeostasis in infected host cells.
How phosphorylation of STING and MAVS is regulated and how this post-translational modification is manipulated by viruses remain largely unknown.
Recently, a research group led by Prof. Deng Hongyu from the Institute of Biophysics, Chinese Academy of Sciences (CAS), identified a host protein phosphatase PPM1G that negatively regulates STING- and MAVS-mediated innate immune responses, and showed that Kaposi's sarcoma-associated herpesvirus (KSHV) hijacks PPM1G for immune evasion via its tegument protein ORF33.
The study was published online in Science Advances on Nov. 20.
Prof. Deng's group has previously shown that ORF33, a conserved tegument protein of herpesviruses, is essential for virion assembly of gammaherpesviruses. However, it is not yet known whether ORF33 can mediate immune evasion function.
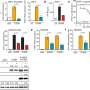
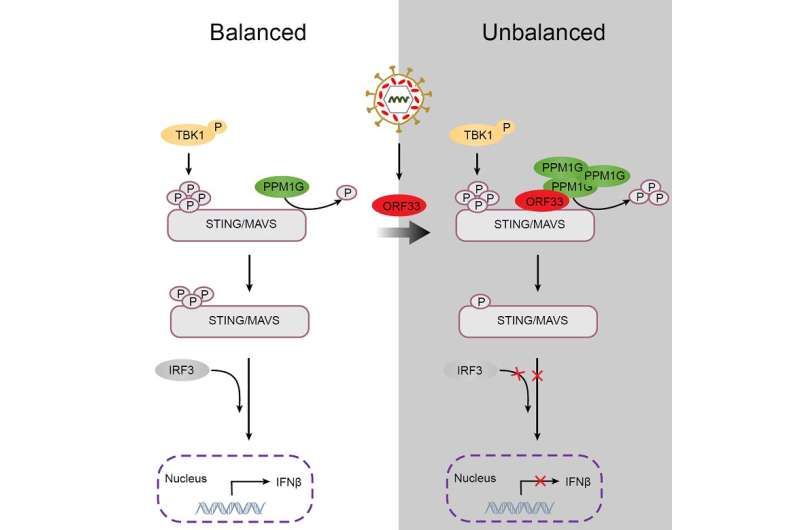
In this study, the researchers first constructed an ORF33-null KSHV mutant. Results indicated that ORF33 inhibited the host innate immune responses by affecting the functions of the adaptor proteins STING and MAVS.
Mechanistically, the expression of ORF33 markedly decreased the phosphorylation levels of STING and MAVS. Interestingly, in the in vitro phosphatase assay, only ORF33 purified from mammalian cells, but not ORF33 purified from prokaryotic cells, reduced the phosphorylation levels of STING and MAVS, indicating that ORF33 may utilize host protein phosphatase(s) to dephosphorylate STING and MAVS.
Using coimmunoprecipitation and mass spectrometry analysis, the researchers identified a host protein phosphatase PPM1G that is associated with ORF33. PPM1G purified from prokaryotic cells directly dephosphorylated STING and MAVS in the in vitro phosphatase assay. Moreover, ORF33 enhanced the interaction between PPM1G and STING or MAVS. These results demonstrated that ORF33 recruits PPM1G to dephosphorylate STING and MAVS, thus inhibiting their activation.
Furthermore, PPM1G inhibited host IFNβ response; consistently, PPM1G knockdown or knockout enhanced host defense against DNA and RNA viruses. These results indicated that PPM1G negatively regulates both DNA and RNA sensing pathways.
This study identified a host protein phosphatase PPM1G that serves as a negative regulator to restrict excessive activation of antiviral innate immunity. It also revealed a novel strategy of viral immune escape: KSHV tegument protein ORF33 recruits PPM1G to dephosphorylate STING and MAVS, thereby inhibiting IFN production and antiviral responses.
More information: Kuai Yu et al. PPM1G restricts innate immune signaling mediated by STING and MAVS and is hijacked by KSHV for immune evasion, Science Advances (2020). DOI: 10.1126/sciadv.abd0276
Journal information: Science Advances
Provided by Chinese Academy of Sciences