This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Control over friction, from small to large scales
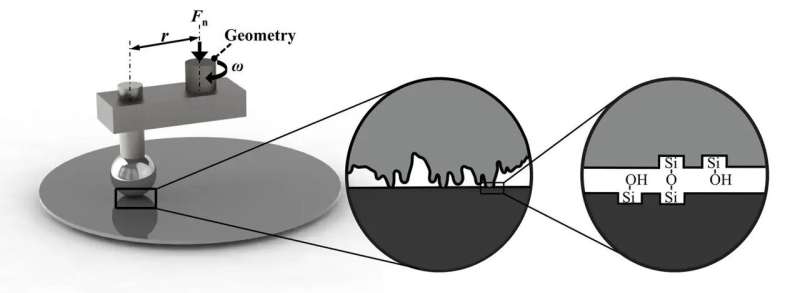
Friction is hard to predict and control, especially since surfaces that come in contact are rarely perfectly flat. New experiments demonstrate that the amount of friction between two silicon surfaces, even at large scales, is determined by the forming and rupturing of microscopic chemical bonds between them. This makes it possible to control the amount of friction using surface chemistry techniques. This research has been published in Physical Review Letters.
"There is a lack of quantitative understanding of friction, despite its crucial role in tackling challenges as diverse as predicting earthquakes and reducing energy consumption in mechanical devices," says Ph.D. researcher Liang Peng, who conducted the research project. This is no small matter: Friction is estimated to be responsible for more than 20% of our global energy consumption. Controlling friction in machinery is also important for reducing material wear and increasing positioning precision.
Peng worked together with other researchers of the Institute of Physics and the Van 't Hoff Institute of Molecular Sciences at the University of Amsterdam as well as the Advanced Research Center for Nanolithography (ARCNL). The research is part of an ongoing collaboration to investigate how large-scale friction emerges at a microscopic level.
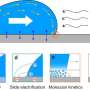
In recent years, new research methods have allowed researchers to zoom in on what exactly happens when two surfaces make contact and slide over one another. Crucially, surfaces are never perfectly smooth. At the scale of a nanometer, one-billionth the size of a meter, they look like mountainous landscapes with pronounced peaks and valleys.
Previous experiments and numerical simulations have demonstrated that at this small scale, friction is largely determined by the formation and rupturing of bonds between surface atoms. This is affected not just by the roughness of the sliding surfaces, but also by which atoms or molecules (such as water) are present at the interface.
"We decided to extend and apply these nanofriction mechanisms to larger, industrially relevant scales," explains Peng. Using a special instrument called a rheometer, the researchers studied how the amount of friction between a relatively rough silicon ball and a smooth silicon wafer depends on the density of microscopic chemical bonds at the interface. Silicon (Si) is a particularly interesting material to study thanks to its widespread use in the semiconductor industry. Its abundance in the Earth's crust also makes it relevant to the study of earthquakes.
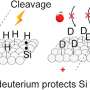
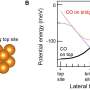
After cleaning the surfaces of contaminants, the researchers discovered that much less force is needed to slide the ball over the wafer—in other words, there is less friction—when the surfaces are dried for longer in pure nitrogen gas. Further experiments showed what happens at the level of atoms: longer drying reduces the number of hydroxyl (OH) groups exposed at the silicon surface. When brought into contact with another silicon surface, the presence of these groups results in the formation of silicon–oxygen–silicon (Si–O–Si) bonds between the two surfaces.
The research demonstrates that there is a striking relation between the friction force measured at large scales and the density of microscopic Si-OH groups present on the two silicon surfaces before contact, which controls the number of Si-O-Si bonds made during contact. The density of these chemical bonds is regulated by setting the length of time for which the cleaned surfaces are dried. Excitingly, this means that it is possible to predict and control the friction force between silicon surfaces.
"Our result is quite remarkable because it demonstrates a quantitative understanding of macroscopic friction from first principles. Our findings can thus bridge the knowledge gap that hampers understanding-based control over friction," concludes Liang.
More information: Liang Peng et al, Controlling Macroscopic Friction through Interfacial Siloxane Bonding, Physical Review Letters (2023). DOI: 10.1103/PhysRevLett.131.226201
Journal information: Physical Review Letters
Provided by University of Amsterdam