This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Discovery reveals lipid-signaling microdomains in cells
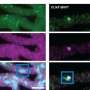
![Medically important phospholipids are observed in cellular condensates. [Top] PIP2 (green), a phospholipid involved in cell signaling, is observed in nuclear speckle condensates (red). [Bottom] PIP3 (green), another signaling phospholipid, is observed in stress granule condensates (red). Images were taken of human cells on a tissue culture plate. . Credit: Dr. Jason Dumelie a, Proteins used in the metabolomics studies. Modular domains (gray) and disordered regions (pink) are displayed over disorder scores (y axis) for each amino acid (x axis). Brackets indicate the protein region attached to mCherry. Disorder scores were determined by IUPred3 (ref. 67). RRM, RNA recognition motif. b, Condensate metabolomics procedure. Condensate-forming proteins, in the presence of metabolites, were stimulated to form condensates by the addition of RNA. After a brief incubation, condensate and aqueous phases were separated by centrifugation and analyzed by LC–MS. c, RNA stimulates nucleocapsid and MED1 phase separation. Nucleocapsid (30 μM; top, red), MED1 (30 μM; middle, red) and HNRNPA1 (30 μM; bottom, red) were incubated (10 min, 25 °C) in the absence (left) or presence (right) of RNA (150 nM) and imaged by confocal microscopy. Although the addition of RNA did not impact the number of HNRNPA1 condensates, there were increased numbers of nucleocapsid and MED1 condensates after RNA addition; scale bar, 5 μm (n = 2). d, Nucleocapsid is enriched in the condensate phase. Coomassie G-250 dye-stained gels were used to assess whether centrifugation-separated nucleocapsid concentrates in a distinct phase. Nucleocapsid is almost undetectable in input (left) and aqueous (center) fractions but is readily detected in the condensate fraction (right). The arrow indicates the expected mCherry–nucleocapsid location (n = 2). e, Condensate detection in postcentrifugation fractions. Nucleocapsid (red) in the aqueous (left) and condensate (right) postcentrifuge fractions was imaged by fluorescence microscopy. Fractions were diluted in LC–MS-compatible buffer (1:4) before imaging. Condensates are only visible in the condensate fraction; scale bar, 5 μm (n = 2). Discovery reveals lipid-signaling microdomains in cells](https://scx1.b-cdn.net/csz/news/800a/2023/discovery-reveals-lipi.jpg)
Important signaling molecules called phospholipids are active throughout cells in small compartments called condensates, rather than functioning primarily in cell membranes as previously thought, according to a study from researchers at Weill Cornell Medicine. The finding helps open a new avenue of investigation in cell biology and may also be relevant to the study of neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) and Alzheimer's disease.
Condensates in cells, also called biomolecular condensates, behave like oil drops within water. They are made of proteins, and often RNA molecules, that have weakly conglomerated to form distinct globules in the cell. These globules form compartments with chemical properties that differ from those of the surrounding, watery interior of the cell.
There are many different kinds of condensates, and their apparent functions include concentrating proteins that work together to support cellular processes and sequestering RNAs when cells are under stress. Condensates are now also seen as potential sites for the formation of abnormal protein aggregates found in neurodegenerative disorders, including ALS, Alzheimer's, Parkinson's and Huntington's disease.
In the study, published in Nature Chemical Biology, the researchers found evidence that condensates frequently contain phospholipids along with their well-known protein and RNA constituents. These condensates often contain enzymes that act on phospholipids, suggesting a previously overlooked role for condensates as phospholipid signaling centers. The scientists also found that they could manipulate the numbers and properties of condensates by altering phospholipid levels.
"This is a basic science discovery that shows major sites of lipid signaling in cells," said study senior author Dr. Samie Jaffrey, the Greenberg-Starr Professor in the Department of Pharmacology at Weill Cornell Medicine.
The study's first author is Dr. Jason Dumelie, an instructor in pharmacology and a member of the Jaffrey lab at Weill Cornell Medicine. The study also included a collaboration with the laboratory of analytical chemistry expert Dr. Steven Gross, a professor of pharmacology at Weill Cornell Medicine.
Although scientists increasingly see condensates as important in health and disease, they know relatively little about condensates' molecular constituents apart from proteins and RNAs.
In the study, Drs. Jaffrey and Dumelie and their colleagues focused on small organic molecules in cells that are often involved in biochemical processes and are known as metabolites. The researchers made lab-dish versions of common condensates and surrounded them with an ordinary cellular mix of hundreds of metabolites.
With the help of Gross lab member Dr. Qiuying Chen, an associate professor of research in pharmacology, they used a technique called mass spectrometry to catalog metabolites that became more concentrated inside the condensates.
To their surprise, they found that condensates formed from different protein conglomerations nevertheless tended to attract similar sets of metabolites. Prominent among these metabolites were fat-related molecules called lipids, particularly phospholipids.
Phospholipids are key constituents of cell membranes that work as signaling molecules in a variety of cell processes, including immune, stress-response, and cognitive functions.
"Normally, if you ask scientists where phospholipids reside in cells, they'll say in cell membranes," Dr. Dumelie said. "But as our study shows, they are also in these condensates."
The finding helps explain prior research, which found that enzymes known to mediate phospholipid signaling are mysteriously present in condensates. However, the implications could go well beyond basic cell biology, given the links between condensates and protein aggregation in neurodegenerative disorders.
"We found in our study that by adding phospholipids, we could substantially change the properties of one of the condensates we looked at," Dr. Dumelie said. "That suggests the possibility—which we're investigating now—of using such lipids to alter condensates to prevent the formation of toxic protein aggregates in neurodegenerative disorders."
More information: Jason G. Dumelie et al, Biomolecular condensates create phospholipid-enriched microenvironments, Nature Chemical Biology (2023). DOI: 10.1038/s41589-023-01474-4
Journal information: Nature Chemical Biology
Provided by Weill Cornell Medical College