This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Cellular study reveals a lipid-mediated, inter-organelle biogenesis network
Researchers from Telethon Kids Institute and The University of Western Australia have developed a new technique to see inside cells with unprecedented detail, revealing a complicated web of interactions that provides new insights into how cells stay healthy.
According to lead researcher Professor Aleksandra Filipovska, Lou Landau Chair in Child Health Research at Telethon Kids Institute and UWA, the knowledge gained could pave the way for new treatments for a range of currently incurable diseases—in particular, debilitating mitochondrial diseases which affect up to 1 in 5,000 babies born in Australia.
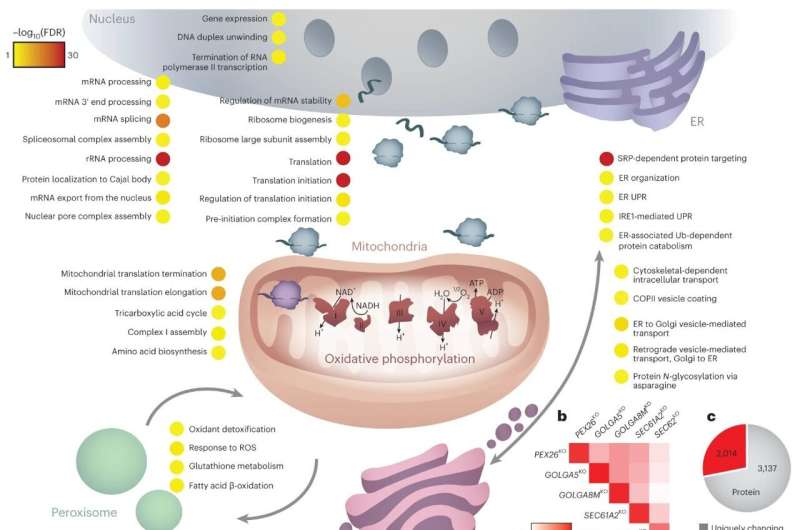
Much like our body needs organs to function, each of our cells has inner "organs" called organelles. Within each cell, these organelles collaborate, with each performing specific functions: the mitochondria produce energy, the rough endoplasmic reticulum makes and folds proteins that are exported from the cell, the Golgi apparatus processes proteins and fats, and the peroxisome deals with the destruction of fats no longer needed by the cell.
Professor Filipovska said it was already well known that the structures and functions of organelles in cells depended on each other for cell health, however, until now these relationships had not been systematically explored.
To understand more about how their interactions influence cellular health, she and her team used advanced cellular biology techniques to visualize organelle structure and function in three dimensions.
In a multi-year study detailed today (Dec. 21) in Nature Cell Biology, they used a powerful imaging technique—focused ion beam scanning electron microscopy—to watch what happens within cells deliberately engineered to harbor mutations that damage organelles.
"We showed that if one of the organelle team members isn't doing their job, it can cause trouble for the whole cell—and that has implications for how diseases may be understood and treated," Professor Filipovska said.
In particular, organelles rely on specific types of fats (ether-glycerophospholipids) to function properly. The study found that when certain genes related to these fats were turned off in cells, it caused problems in various organelles.
"These gene changes led to a decrease in specific fats in the cells, affecting the structure and function of mitochondria—the cell's powerhouses," Professor Filipovska said.
"Additionally, disrupted fats impacted how different organelles communicated and behaved. Certain cells, when lacking these fats, showed issues in their Golgi, a structure involved in processing fats. This led to changes that affected overall cell health."
The study explored potential solutions and found that by providing the cells with specific fat-building blocks, they could partially fix the issues.
"Our work has identified that specific lipids can rescue the function of the powerhouses in the cell and improve their communication with the rest of the cell," Professor Filipovska said.
"This finding has important implications for treatment of diseases caused by diminished energy supplies."
Professor Filipovska said the study's findings had the potential to make a huge difference to clinicians' ability to diagnose disease and could pave the way for therapies for mitochondrial diseases and other diseases caused by cellular mutations, which have lacked effective treatments until now.
"For families affected by mitochondrial disease it really opens the way to hope—something they haven't really had in the 35 years since these diseases were initially discovered," she said.
The work is now set to be expanded into a full research program. Using a newly announced Investigator Grant—awarded to Professor Filipovska by the National Health and Medical Research Council last week—the team will build on the study's findings to address the prolonged and complex diagnostic process for mitochondrial diseases and develop new treatments.
About mitochondrial disease
Mitochondrial diseases—most often caused by mutations in mitochondrial DNA which deplete energy in the body—are progressive multi-system disorders that affect up to 1 in 5,000 live births. There are no cures and very limited treatments available, with patients mostly supported by measures including palliative surgeries or anti-epileptic drugs.
The diseases can be debilitating and devastating, causing diminished growth, brain and nervous system failure, loss of hearing, motor function impairments, liver dysfunction and heart failure that require constant medical care and can result in premature death. Mitochondrial diseases most often affect young babies and children.
More information: Richard G. Lee et al, Quantitative subcellular reconstruction reveals a lipid mediated inter-organelle biogenesis network, Nature Cell Biology (2023). DOI: 10.1038/s41556-023-01297-4
Journal information: Nature Cell Biology
Provided by Telethon Kids Institute