This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Breakthrough in the synthesis of artificial cells
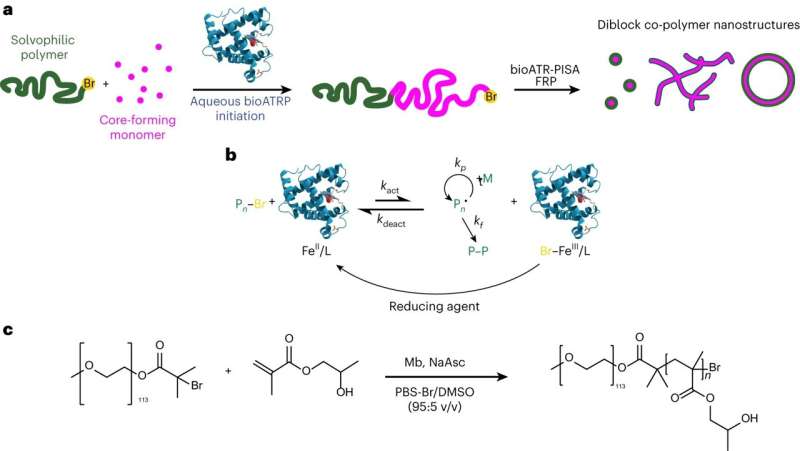
A study published in Nature Chemistry reveals a remarkable leap in the synthesis of artificial cells using synthetic materials, which was achieved by an international team led by Dr. Andrea Belluati, Prof. Nico Bruns (both TU Darmstadt) and Dr. Sètuhn Jimaja (University of Fribourg).
These cells, crafted through a process called biocatalytic polymerization-induced self-assembly (bioPISA), represent a significant advancement in the field of synthetic biology.
Artificial cells are microscopic structures that emulate the properties of living cells. They represent important microreactors to enhance chemical reactions and for molecular systems engineering, act as hosts for synthetic biology pathways, and are important tools to study the origin of life.
The team developed an enzymatic synthesis of polymeric microcapsules and used them to encapsulate the soluble contents (i.e., the cytosol) of bacterial cells, thereby creating artificial cells with the ability to produce a range of proteins on their inside, including a fluorescent protein, the structural protein actin to craft a cytoskeleton-like structure, and the enzyme alkaline phosphatase to imitate the biomineralization process found in human bones.
The expression of proteins not only mimics one of the fundamental properties of living cells but also showcases the potential of these artificial cells in various applications, from drug delivery to tissue engineering.
"Our study bridges a crucial gap in synthetic biology, merging the world of synthetic materials with enzymatic processes to create complex, artificial cells, just like real cells," says Belluati. "This opens up new horizons in creating cell mimics that are not just structurally similar to biological cells but functionally competent as well."
Bruns adds, "Enzymatic radical polymerizations are the key to creating these artificial cells. Enzymes synthesize polymers that self-assemble during the polymerization into nano- and micro-sized polymer capsules. This is a very simple yet efficient way to prepare the artificial cells. In future work, we aim to use proteins expressed in the artificial cells to catalyze further polymerizations, thereby mimicking the growth and replication of natural cells."
More information: Andrea Belluati et al, Artificial cell synthesis using biocatalytic polymerization-induced self-assembly, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01391-y
Journal information: Nature Chemistry
Provided by Technische Universitat Darmstadt