This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers tune the speed of chirality switching
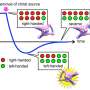
Chiral molecules can have dramatically different functional properties while sharing identical chemical formulae and almost identical structures. The molecular structure of two types of a chiral molecule—so-called enantiomers—are mirror images of each other where one cannot be superposed on the other any more than your right hand can fit front-to-back on the left. While a lot of chiral molecules are traditionally considered fixed as left- or right-handed, chiral molecules based on helices are known to be able to switch in response to changes in their environment.
Now, researchers led by Shigehisa Akine at Kanazawa University have demonstrated how environmental changes can also accelerate or decelerate this chiral inversion process, providing a novel time-programmable switchable system. The research is published in the journal Science Advances.
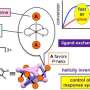
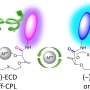
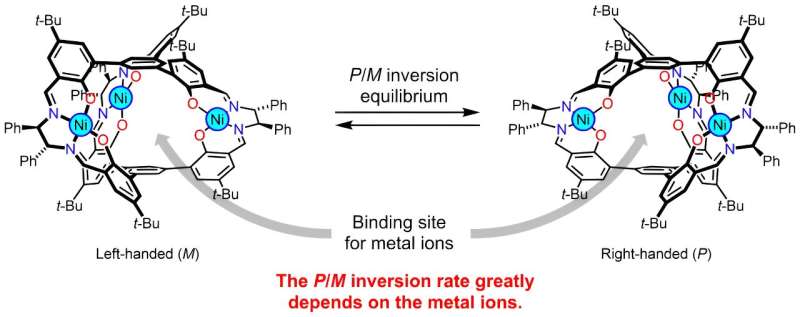
The researchers focused their study on metallocryptand (R6)-LNi3, an organic molecule featuring metal atoms in a cage-like molecular structure that can exist in one of two possible forms described as the P or M type (right- and left-handed, respectively) (Fig. 1). In its pure form (R6)-LNi3 has a preferred ratio of P type to M type of 12:88.
Starting from a 50:50 ratio, the molecules will flip between one form and the other with a preference for flipping towards the M type to meet that ratio. The researchers measured this change in ratio using NMR and circular dichroic spectroscopy. However add an alkali metal into the cage cavity and this preference can change.
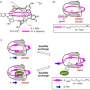
By adding alkali metal ions to the solution of the (R6)-LNi3 the researchers could confirm that the metal ions readily bound to the metallocryptand from the changes in the spectroscopic signatures of the molecules. In addition the bound ion also shifted the preferred ratio by a margin and with a speed that depended on which alkali metal was used.
The researchers attribute the different rates and ratios to differences in binding constants not just between the metal ion and the two forms of the molecule but also a virtual binding constant for the molecule transitioning between the two. The binding between cesium ion and the P type molecule was more than 20 times greater than that with the M type so the solution eventually switched to a higher proportion of the P type with a P:M ratio of 75:25 over the course of 21 hours.
The final ratio with rubidium ion was similarly bias to the P type reaching a slightly lower ratio of 72:28 but in just 100 minutes. With potassium ion the equilibrium ratio was lower again at 68:32 but reached within just a minute, three orders of magnitude faster than for the cesium ion (Fig. 2). The researchers attribute this speed to the large virtual bonding constant with the transitioning molecule.
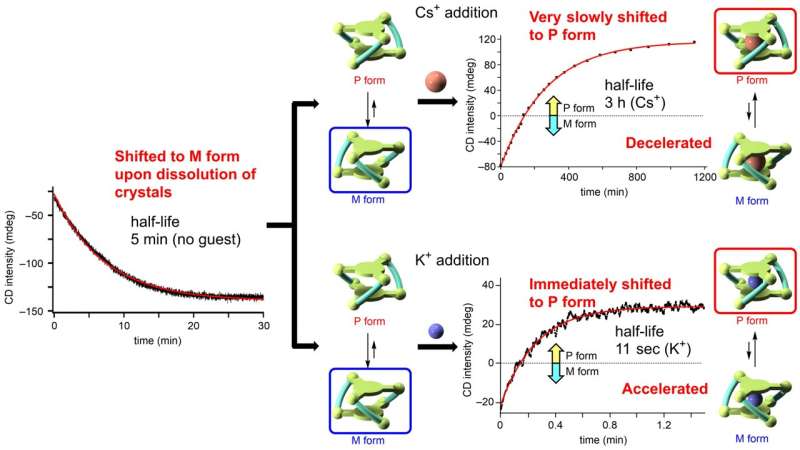
With smaller ions—lithium and sodium ions—the preferred molecular type did not actually change but the final ratio was reached much faster. It is the first time researchers have demonstrated that such chiral inversion can be sped up and slowed down by tuning the molecules environment.
"This research can provide a new insight into the development of an on-demand time-programmable molecular clock for new generation chemical technologies," conclude the researchers, citing as possible future technologies a memory device with a controllable chemical information processing time, as well as chiral sensors whose selectivity is invertible depending on the situations.
More information: Sk Asif Ikbal et al, Acceleration and deceleration of chirality inversion speeds in a dynamic helical metallocryptand by alkali metal ion binding, Science Advances (2023). DOI: 10.1126/sciadv.adj5536
Journal information: Science Advances
Provided by Kanazawa University