This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Researchers propose an organic-solvent-free method for producing nanosized vaterite
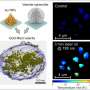
Vaterite is one of the three forms of calcium carbonate, along with calcite and aragonite. Nanosized vaterite is valuable for various applications, such as drug delivery, cosmetics, and bone defect filling, owing to its biocompatibility, high porosity, solubility, and large specific surface area.
Vaterite is not commonly found in nature as it transforms into calcite over time. In laboratory settings, organic solvents are used to prevent its recrystallization and hinder particle growth. However, the solvents are expensive, highly toxic, and generate significant waste, making them harmful both to humans and the environment. Therefore, there is an urgent need for a method that circumvents these challenges, is cost-effective, and results in environmentally friendly synthesis of vaterite.
Addressing these concerns associated with vaterite production, a team of researchers from Korea Maritime & Ocean University, led by Professor Myoung-Jin Kim of the Department of Environmental Engineering, has reported an indirect carbonation method that uses seawater to produce nanosized vaterite. Their work was published in Ultrasonics Sonochemistry.
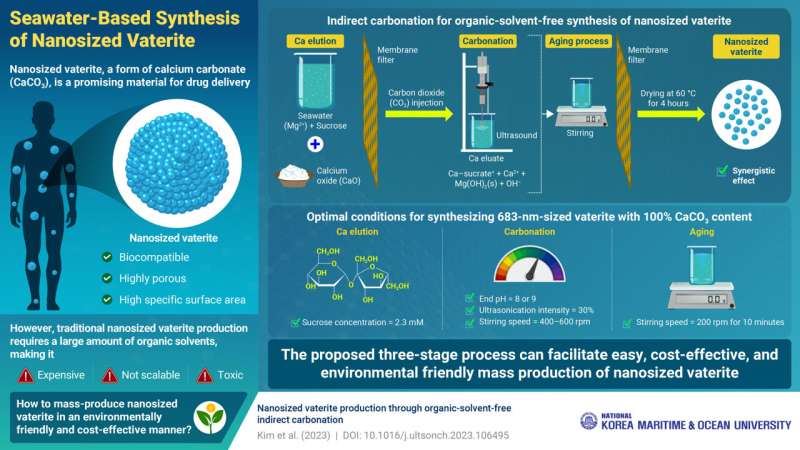
Speaking about the method developed by them, Prof. Kim says, "The entire process comprises of three stages: calcium elution, carbonation, and aging." In the calcium elution stage, a solution containing seawater and sucrose is mixed with calcium oxide. The magnesium ions present in seawater facilitate the dissolution of calcium into the solution, leading to the release of free Ca2+ ions. Sucrose forms a complex with Ca2+ ions.
The eluted Ca2+ ions are then reacted with injected carbon dioxide in the carbonation stage, resulting in the formation of calcium carbonate (CaCO3) as a solid precipitate. The growth of the CaCO3 particles is subsequently suppressed by ultrasonic vibrations generated by a sonifier. Subsequently, the mixture undergoes aging, where CaCO3 particles are further reduced in size, resulting in the formation of nanosized vaterite.
Each step of the proposed method contributes to vaterite production and particle size reduction, with optimal conditions resulting in nanosized particles with 100% vaterite. The size and content of vaterite are highly sensitive to the sucrose concentration used in the calcium elution stage. The researchers further report that the ideal concentration was found to be 2.3 mM, which produced a large amount of free Ca2+ ions, without increasing the viscosity of the solution.
In the carbonation step, controlling the end pH, ultrasonic intensity, and stirring speed is critical. The researchers determined that stopping the carbonation reaction at pH levels of 8 or 9 resulted in 100% vaterite content, while ultrasonication at 30% intensity and stirring at 400–600 rpm produced nanosized particles. Moreover, stirring at a speed of 200 rpm for 10 minutes was optimal for aging.
The outcome of these steps is the production of pure vaterite with particle sizes of 683 nm, achieved without organic solvents. "These findings highlight the possibility of mass-producing nanosized vaterite using seawater, which is an environmentally friendly solvent. The proposed method can be highly advantageous from economic and environmental viewpoints for the mass production of nanosized vaterite, without requiring a substantial amount of organic solvent," concludes Prof. Kim.
More information: Sehun Kim et al, Nanosized vaterite production through organic-solvent-free indirect carbonation, Ultrasonics Sonochemistry (2023). DOI: 10.1016/j.ultsonch.2023.106495
Provided by National Korea Maritime and Ocean University