This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
How to develop bio-inspired catalysts

Victor Mougel is an absolute fan of nature, not only because he grew up on a farm, spends a lot of time outdoors with his wife and children, and sometimes rides up and down Swiss mountains on his road bike. He also believes that no chemist can hold a candle to nature.
"Nature is able to carry out extremely challenging reactions in the most efficient manner, it's an amazing source of inspiration," the assistant professor enthuses. His group at ETH Zurich draws inspiration from natural systems at all scales: replicating macroscopic shapes of living organisms but also mimicking natural systems at the micro and molecular level, specifically focusing on enzymes. These highly efficient natural catalysts drive a multitude of reactions in nature.
Learning from 3 billion years of evolution
Catalysis is a process in which certain molecules (catalysts), are used to accelerate reactions and thus transform substances.
"In contrast to nature, chemists often use rare metals as catalysts, an unsustainable source for processes at a global scale," Mougel explains. Most building blocks for chemical production are currently derived from fossil sources, presenting environmental challenges, including the problematic accumulation of carbon dioxide and nitrates. Electrochemistry is an appealing option to sustainably convert back these troublesome molecules.
"A key element of this approach is designing new electrocatalysts that enable this transformation with high activity and selectivity, and for sustainability, only use earth-abundant elements," he says.
Here, nature's ingenuity shows the way: "For over three billion years, nature has been developing enzymatic catalysts to efficiently utilize abundant molecules like N2 and CO2, essential compounds for constructing complex molecules and materials," Mougel states enthusiastically. "We can exploit this and develop bio-inspired catalysts that can help to solve our most pressing problems."
Creating artificial leaves and bio-inspired CO2 reduction
For his purpose, Mougel and his group follow two approaches: First, they try to replicate the structure of the active sites of enzymes; second, they imitate functions found in enzymes, striving to reproduce these functions without limiting themselves to structures found in nature.
Mougel's team was able to produce, for instance, an artificial "leaf," as part of a research collaboration.
"Carbon dioxide, one of our most pressing environmental problems, is a stable, oxidized molecule," Mougel points out. "One solution could be to design enzyme-inspired catalysts that efficiently reduce CO2—transferring electrons to the molecule—and thus convert it into useful products. People often forget that CO2 and nitric oxides are not just waste products and a threat to the climate. They are primarily the basic building blocks of life and an important basis material from which useful chemicals can be produced."
That was the idea behind the artificial leaf, he explains: "Instead of converting carbon dioxide and water into oxygen and sugar as natural leaves do, our system produces hydrocarbons using sunlight as the sole energy source."
Furthermore, the group developed efficient catalysts for the reduction of CO2 to formic acid, an important industrial compound. To do this, the group mimicked the active site of the enzyme carbon monoxide dehydrogenase (CODH), which contains two metals.
Controlled production of metal hydrides
Recently, the group focused on a key feature of enzymatic systems: electron transfers. In nature, electron transfers are typically mediated by iron-sulfur clusters. These clusters are essential for most living organisms, being involved in processes such as photosynthesis, mitochondrial energy production, and DNA replication.
"They act as natural electrical wires and transfer electrons across the protein structures, while also being involved in proton transfers and in the activation of small molecules," Mougel notes.
Synthetic iron-sulfur clusters could be exploited to design better electrocatalytic systems, he explains: "We could prove, for example, that if we combine known catalysts for the reduction of carbon dioxide with iron-sulfur clusters, we could not only strongly enhance their catalytic activity, but also completely change their selectivity."
The group demonstrated that the clusters are promoting a so-called concerted proton-electron transfer (CPET), where a proton and an electron are stored and transferred simultaneously from the cluster to a substrate. Mougel and his group succeeded for the first time in producing a metal hydride in a controlled manner in this way and use this hydride for the conversion of CO2 to formic acid. This constituted the first experimental demonstration of that important concept that is expected to have broad implications for electrocatalysis, since metal hydrides are central intermediates in many catalytic transformations.
Mimicking the electrical wires of nature
Such examples show that understanding natural systems is key. This is why in Mougel's laboratory, application and basic research always go hand in hand. The group has also investigated the fundamental redox properties of iron-sulfur clusters in detail.
"What's exciting is the following: If you want to convert CO2 into industrially useful compounds such as long-chain hydrocarbons, ethylene, or ethane, up to 14 electron reductions are necessary. However, all biological CO2 reductase enzymes are restricted to two electron processes," Mougel explains, "but the nitrogenase enzyme complex has an iron protein with an iron-sulfur cluster that can, in principle, bypass this limitation, although this does not occur in biological systems. However, an artificial model to study this in more detail had been missing until now."
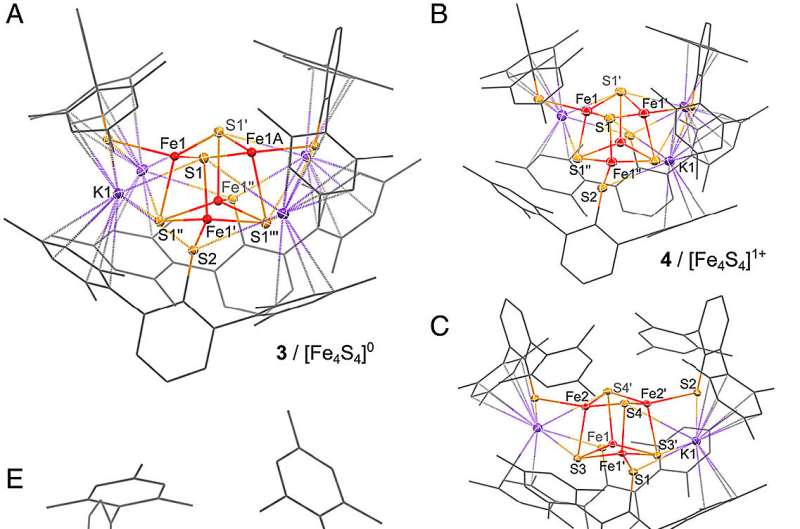
Mougel and his group have succeeded for the first time in isolating and stabilizing such extremely reduced iron-sulfur, and finally synthesizing and characterizing a complete series of so-called iron-sulfur cubane redox clusters in all oxidation states. This allowed a thorough analysis of the varying structural and electronic properties.
In the next step, the group was able to show that even small changes in the environment of these clusters can have a major impact on their dynamics and redox potentials. This allows the generation of extreme reducing potentials—so facilitated oxidation and reduction—in situ and on-demand (gating concept).
For his research, in particular for his contributions in the field of iron-sulfur clusters, Victor Mougel is being awarded the 2023 Ruzicka Prize—an honor that means a lot to him, but which—as he emphasizes—he is accepting only as a representative of many minds.
"I would like to thank my team, because ultimately, none of these research successes would have been possible without their commitment. The people in my group are the driving force behind this research and an important motivation for me to continue setting up projects together."
Journal information: Proceedings of the National Academy of Sciences
Provided by ETH Zurich