This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists shed light on potential breakthrough biomedical molecule
Scientists from the Department of Energy's SLAC National Accelerator Laboratory have gained valuable insights into producing nitroxide, a molecule with potential applications in the biomedical field. While nitric oxide (NO) has long been on researchers' radar for its significant physiological effects, its lesser-known cousin, nitroxide (HNO), has remained largely unexplored.
The study, published recently in the Journal of the American Chemical Society, was born out of a joint endeavor between teams at SLAC's Linac Coherent Light Source (LCLS) X-ray laser and Stanford Synchrotron Radiation Lightsource (SSRL).
Nitroxide has many of the same physiological effects of nitric oxide—such as its ability to fight germs, prevent blood clots, and relax and dilate blood vessels—with additional therapeutic properties, such as efficacy in treating heart failure, as well as more potent antioxidant activity and wound healing. However, it is not a chemically long-lived species so methods that enable its targeted delivery are key to future biomedical applications.
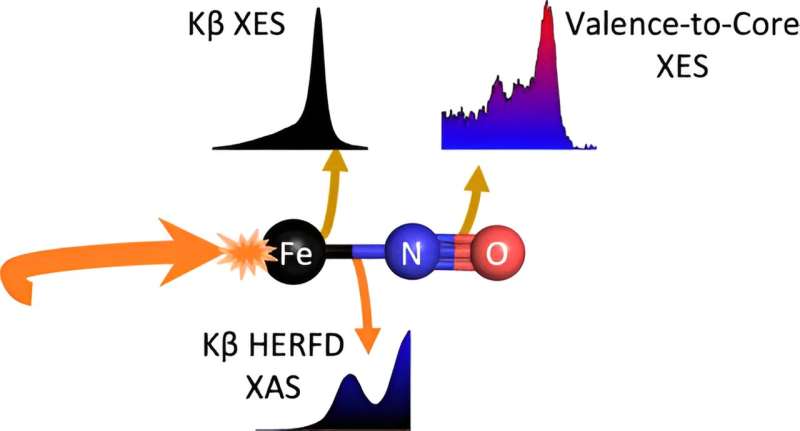
To address this challenge, the team focused on a unique molecule, an iron-nitrosyl complex (Fe-NO). Their research aimed to understand the intricate properties of the Fe-NO bond, both before and after light exposure, to navigate the complexities of nitroxide production. They discovered that by exposing this molecule to optical light, they could break its bond, potentially producing nitroxide.
"Although this research is fundamental in nature, the hope is that other researchers can take what we learn from this molecule and build therapeutic technologies off of it by optimizing similar molecules for medicine," said SLAC scientist and collaborator Leland Gee. "The idea would be to get a molecule that releases HNO in the body where it is needed and shine light on it to release it for the therapeutic properties."
One of the challenges the team faced was the ambiguous distribution of electrons between the iron atom and the nitrosyl ligand—a molecule or ion that binds to a central metal atom or ion—in the Fe-NO complex, which limits how much information can be gained using traditional methods. The scientists employed advanced X-ray spectroscopic techniques at SSRL that allowed them to peer deeper into the chemical properties of the molecule and its bond, providing a more complete picture of the Fe-NO system and how it responds to light.
To follow up, the scientists plan to further explore the intricacies of the bond-breaking process and how to optimize the production of nitroxide or nitric oxide. They are also considering replacing iron with other metals to better understand the photoproduction process.
"In this research we understand the starting molecule and its final products after shining light on it," Gee said. "There are still a lot of nuances in the actual bond-breaking and release of nitroxide from this molecule that need to be explored. What step in the process decides the release of nitroxide instead of nitric oxide? How can we structurally tune the system to produce either molecule?"
This work helps build an understanding of which properties to monitor in future experiments at LCLS, where scientists will be able to take real-time snapshots of the nitroxide photogeneration process.
"The information we gained highlights the power of this approach and serves as a blueprint for future studies on these and similar molecules in the future that will extend to studies at the LCLS," Gee said.
The research holds promise for the medical community and patients who might benefit from its future applications.
"Although we are still far away from using light on these molecules to treat serious cardiovascular conditions, fundamental insights in these molecules lay substantial groundwork for applied research in the future," Gee said. "This may lead to entirely new ways to use light to treat cardiovascular conditions, microbial infections, cancer and other health conditions."
More information: Leland B. Gee et al, Unraveling Metal–Ligand Bonding in an HNO-Evolving {FeNO}6 Complex with a Combined X-ray Spectroscopic Approach, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c04479
Journal information: Journal of the American Chemical Society
Provided by SLAC National Accelerator Laboratory