This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Just add salt: Researcher discovers a safe, simple way to make disinfectants work better
A chemical engineer at the University of Alberta has developed a disinfectant that is more effective than purely alcohol-based products, just by adding salt.
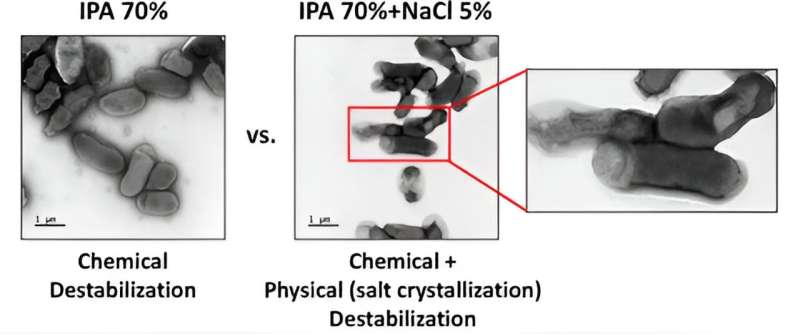
Typical disinfectant solutions of 70% alcohol to 30% water have severe limitations, says Hyo-Jick Choi of the Department of Chemical and Materials Engineering.
One problem with alcohol is it evaporates too quickly from surfaces such as countertops, glass and stainless steel, reducing its effectiveness against tough pathogens such as Clostridium difficile. Often referred to as C. difficile, the bacteria cause mild to severe diarrhea and intestinal conditions, and are the most frequent cause of infectious diarrhea in hospitals and long-term care facilities in Canada.
Another limitation that is fast becoming a major challenge in public health is that low exposure of pathogens to alcohol increases their chances of mutating and becoming resistant.
"Think about the typical spray bottle," says Choi. "It may be originally formulated to contain 70% alcohol, but evaporation starts happening as soon as it hits the air and even during storage in a container.
"The percentage of alcohol on the surface you're spraying is lower, so it's not easy to expose pathogens to the alcohol over time."
Environmental conditions such as temperature and air circulation can also increase the rate of evaporation, he adds.
Adding salt to the mix in quantities above 2.5% weight per volume can make a huge difference. But it has nothing to do with the chemical properties of the salt itself, says Choi. Rather, as the alcohol and water in the disinfectant evaporate, the salt recrystallizes and grows, physically destroying the cell walls of pathogens.
"By adding salt, we can add another mechanism—physical destruction—to the chemical destruction, and we can cause the rapid inactivation of pathogens faster and more efficiently, without any concern about mutations or structural difference."
Salt, or sodium chloride, is cheap and safe, he says, unlike toxic chemicals such as quaternary ammonium and chlorine compounds that are sometimes added to disinfectants.
Choi and his team have tested their salt-based disinfectant, which contains 5% weight per volume salt in 70% isopropanol, on "multiple viruses and bacteria" and demonstrated superior results to alcohol-based solutions. Those results are published in the journal ACS Nano.
Choi has had previous success developing protective face masks, using salt recrystallization to deactivate airborne pathogens such as coronavirus.
Because his salt-infused disinfectant is a safer alternative, Choi says there will be no significant issues in obtaining government approval for the disinfectant formulation technology. He is now seeking a patent to market the product along with colleagues at Yonsei University in South Korea.
More information: Euna Oh et al, Highly Effective Salt-Activated Alcohol-Based Disinfectants with Enhanced Antimicrobial Activity, ACS Nano (2023). DOI: 10.1021/acsnano.3c03315
Journal information: ACS Nano
Provided by University of Alberta





















