This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
A mechanism that controls the genomic structure during early differentiation into neuroectoderm
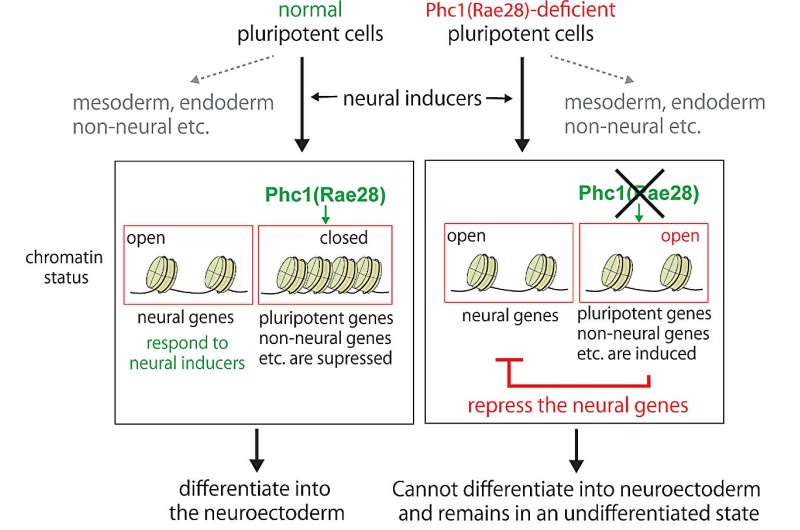
A group of scientists have discovered that a one of the components of the polycomb-type of transcriptional repressor complex (PRC), Phc1 (Rae28), regulates the chromatin state of cells and plays a critical role in the process of undifferentiated cells turning into neural progenitor cells (fig. 1). The research from the Nara Institute of Science and Technology (NAIST), the National Cardiovascular Center, and Japanese Red Cross Kinki Block Blood Center has been published in the October 20, 2023, issue of the journal iScience.
In the initial stages of embryonic stem cell (ES cell) and iPS cell differentiation, the cells started to be categorized into groups such as ectoderm, mesoderm and endoderm. The process requires specific molecules (called "neural inducers") that signal the cells to become neuroectoderm. As cells cannot go back to their undifferentiated state once they have differentiated, their genome structure was believed to significantly change, but the details of this transformation were not clear.
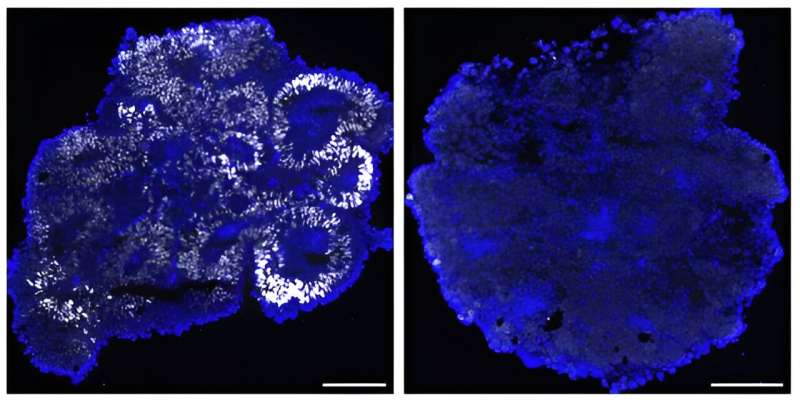
The research team systematically made knockout cells of the PRC genes and concluded that the protein Phc1 (Rae28) is a critical component protein for differentiation into the neuroectoderm. ES cells deficient from Phc1 cannot respond to neural inducers and remained the undifferentiated state (fig. 2). On the other hand, Phc1 can normally differentiate into mesodermal and endodermal cells; therefore, Phc1 is particularly necessary for the neuroectodermal differentiation.
Furthermore, comparison of the chromatin status of normal and Phc1-deficient cells showed that the chromatin structure of the pluripotent genes remained loose in the Phc1-deficient cells, inhibiting the progression of the differentiation. It is therefore apparent that Phc1 governs chromatin states and endows cells with the ability (competence) to react to neural inducers.
In the future, it is expected that this method will be useful in the development of targeted cell differentiation techniques.
More information: Agnes Lee Chen Ong et al, Acquisition of neural fate by combination of BMP blockade and chromatin modification, iScience (2023). DOI: 10.1016/j.isci.2023.107887
Provided by Nara Institute of Science and Technology