This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers devise cleaner, more efficient production of key input for detergents
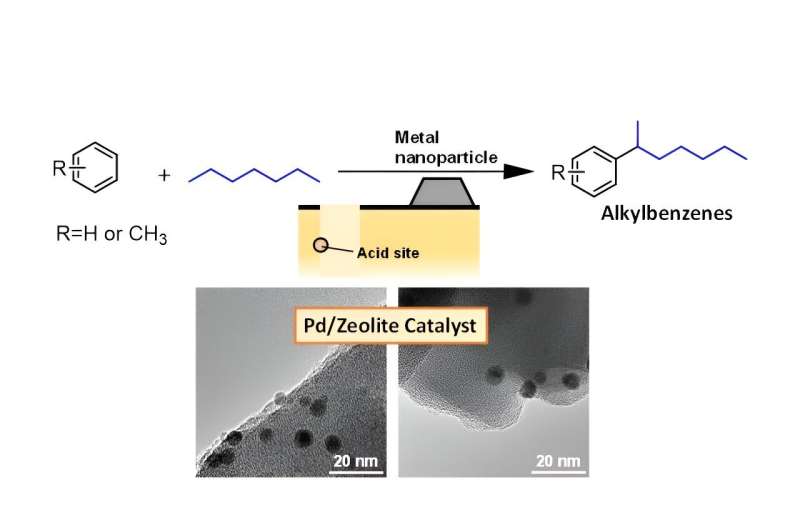
Conventional techniques of generating alkylbenzene, a key input in the production of detergents, produce toxic halogen byproducts, but researchers have devised a new technique that offers a more efficient, cheaper and cleaner manufacturing process of the substance.
A paper describing the process was published in the journal ACS Catalysis on Sept. 6.
In the quest for more efficient and sustainable chemical production processes, researchers have been exploring innovative ways to harness the potential of alkanes—compounds that consist entirely of carbon and hydrogen atoms bonded to one another by carbon-carbon and carbon-hydrogen single bonds.
Methane, ethane, propane and butane are common alkanes, and the main source of alkanes is from crude oil and natural gas. According to the researchers, direct use of alkanes for highly selective chemical transformation is usually difficult because their carbon-hydrogen bonds are highly stable and laborious to break apart.
Soap, toothpaste, laundry detergent and industrial cleaners have a dirty secret: they are all manufactured with alkylbenzenes. Usually, two to three steps are necessary for production of alkylbenzenes because there are additional compounds, called alkylating agents. These agents, produced from alkanes through several steps, are needed to carrying out the alkylation of the benzene—the process of sticking on the alkyl group.
In order to increase the efficiency and lower the cost of this process by reducing the number of steps needed, the researchers investigated the possibility of using simple alkanes directly as the alkylating agent for alkylbenzenes synthesis.
"Not only is it likely to reduce costs, but it should also reduce waste generation and streamlining production, offering a more environmentally friendly alternative to traditional methods," said Professor Ken Motokura, lead author of the study and a chemist with the Department of Chemistry and Life Science at Yokohama National University.
Traditionally, the production of alkylbenzenes involved classical Friedel−Crafts reactions, often resulting in the generation of undesirable byproducts containing toxic halogens. Conventional alkylating agents are alkyl halides, therefore, equimolar amounts of byproducts containing halides are produced.
"By reducing the extra steps, we can reduce generation of those byproducts," Motokura said.
Unlike traditional methods, direct alkylation using alkanes as the alkylating agents produces harmless molecular hydrogen—which is hydrogen gas—as the sole byproduct.
But to achieve this efficiency gain, the development of novel catalysts—the key ingredient in a chemical reaction that kicks it off or speeds it up—was required. The researchers had earlier discovered that palladium nanoparticles (particles of matter just 1-100 nanometers in diameter), became highly efficient catalysts for direct alkylation when situated on the outer surface of H-ZSM-5 zeolites—a type of crystalline material made out of aluminum and silicon with lots of microscopic pores.
These tiny pores are the sites where the chemical reaction is activated.
The process begins with the activation of an alkane on the sites present inside the zeolite pores, which primes the alkane for the addition of a benzene, which is an organic compound in the shape of a ring. This produces the target: alkylbenzene.
During this process, two hydrogen atoms (H) are removed from the alkane and benzene. To regenerate the active sites present inside the zeolite, these hydrogen atoms should be recombined to a hydrogen molecule (H2). A "hydrogen spillover" phenomenon occurs as hydrogen atoms move from the activation sites to the palladium nanoparticles on the outer surface which support the recombination to H2.
According to the researchers, the beauty of direct alkylation lies not only in its efficiency but also in its selectivity—being able to produce one particular desired type of output over other, less desired possible outputs. The researchers were able to achieve a 95.6% selectivity for the desired alkylated products.
Despite the efficiency gains, the overall yield from the process still remains still too low for industrial applications, the researchers said, so the team now aims to tweak their technique to increase its productivity.
More information: Satoshi Misaki et al, Pd Nanoparticles on the Outer Surface of Microporous Aluminosilicates for the Direct Alkylation of Benzenes using Alkanes, ACS Catalysis (2023). DOI: 10.1021/acscatal.3c02309
Journal information: ACS Catalysis
Provided by Yokohama National University