This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
How to protect biocatalysts from oxygen

There are high hopes for hydrogen as the key to the energy transition. A specific enzyme group found in algae and in bacteria can produce molecular hydrogen simply by catalyzing protons and electrons. However, the enzyme group is so sensitive to oxygen that commercial use of the hydrogen produced by this process as a green energy source is not yet possible.
Researchers at the Cluster of Excellence RESOLV and the Research Training Group Microbial Substrate Conversion at Ruhr University Bochum increased the oxygen stability of a hydrogen-producing enzyme by genetically generated channel blockages.
The researchers working with Professor Thomas Happe, head of the Photobiotechnology group, Professor Lars Schäfer, Professor Eckhard Hofmann and Professor Ulf-Peter Apfel published their findings in the journal ChemSusChem on 13 October 2023.
Blocked channels improve oxygen stability
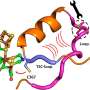
A metalloenzyme with particularly high catalytic turnover rates of molecular hydrogen is called [FeFe] hydrogenase. "The turnover of hydrogen takes place at the active site, the H-cluster, inside the enzyme," explains Happe. "The hydrogen produced inside then travels through channels to exit the enzyme."
"Conversely, if the enzyme is exposed to molecular oxygen, the oxygen also uses specific channels to travel from the enzyme surface up to the H-cluster," adds first author Claudia Brocks. The H-cluster is destroyed at the slightest contact with oxygen and loses the ability to produce any further hydrogen.
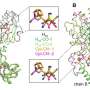
The Bochum research team managed to increase the oxygen stability of the [FeFe] hydrogenase enzyme CpI and explain the oxygen protection effect by using an interdisciplinary combination of methods including site-directed mutagenesis, electrochemistry, X-ray crystallography and molecular dynamics simulations.
"Selective genetic modifications to an enzyme channel altered the hydrogenase enzyme CpI in such a way that we detected a significant increase in oxygen tolerance and oxygen resistance by electrochemical means," explains Brocks. "We used molecular dynamics simulation to study channel modifications. Our analysis revealed blockages in a newly identified dynamic water channel close to the H-cluster."
Happe adds, "The protective effect against harmful oxygen is based on this channel blockage. Oxygen can only penetrate to the H-cluster with difficulty. Local structural modifications can lead to significant changes in protein dynamics."
More information: Claudia Brocks et al, A Dynamic Water Channel Affects O2 Stability in [FeFe] Hydrogenases, ChemSusChem (2023). DOI: 10.1002/cssc.202301365
Provided by Ruhr-Universitaet-Bochum