This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A mysterious blue molecule will help make better use of light energy

Researchers at IOCB Prague are the first to describe the causes of the behavior of one of the fundamental aromatic molecules, which fascinates the scientific world not only with its blue color but also with other unusual properties—azulene. Their current undertaking will influence the foundations of organic chemistry in the years to come and in practice will help harness the maximum potential of captured light energy. Their article appears in the Journal of the American Chemical Society (JACS).
Azulene has piqued the curiosity of chemists for many years. The question of why it is blue, despite there being no obvious reason for this, was answered almost 50 years ago by a scientist of global importance, who, coincidentally, had close ties with IOCB Prague, Prof. Josef Michl.
Now, Dr. Tomáš Slanina is following in his footsteps in order to offer his colleagues in the field the solution to another puzzle. He and his colleagues have convincingly described why the tiny azulene molecule violates the universal Kasha's rule.
This rule explains how molecules emit light upon transitioning to various excited states. If we use the analogy of an ascending staircase, then the first step (the first excited state of the molecule) is high, and each subsequent step is lower and therefore closer to the previous one. The smaller the distance between the steps, the faster the molecule tends to fall from the step to lower levels. It then waits the longest on the first step before returning to the base level, whereupon it can emit light. But azulene behaves differently.
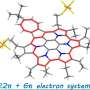
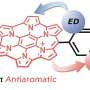
To explain the behavior of azulene, researchers at IOCB Prague used the concept of (anti)aromaticity. Again, simply put, an aromatic substance is not characterized by an aromatic smell but by being stable, or satisfied, if you will. Some chemists even refer to it informally with the familiar smiley face emoticon.
An antiaromatic substance is unstable, and the molecule tries to escape from this state as quickly as possible. It leaves the higher energy state and falls downward. On the first step, azulene is unsatisfied, i.e. antiaromatic, and therefore falls downward in the order of picoseconds without having time to emit light.
On the second step, however, it behaves like a satisfied aromatic substance. And that is important. It can exist in this excited state for even a full nanosecond, and that is long enough to emit light. Therefore, the energy of this excited state is not lost anywhere and is completely converted into a high-energy photon.
With their research, Slanina's team is responding to the needs of the present, which seeks a way to ensure that the energy from photons (e.g., from the sun) captured by a molecule is not lost and that it can be further used (e.g., to transfer energy between molecules or for charge separation in solar cells).
The goal is to create molecules that manage light energy as efficiently as possible. Additionally, in the current paper, the researchers show in many cases that the property of azulene is transferable; it can be simply attached to the structure of any aromatic molecule, thanks to which that molecule gets the key properties of azulene.
Tomáš Slanina adds, "I like theories that are so simple you can easily envision, remember, and then put them to use. And that's exactly what we've succeeded in doing. We've answered the question of why molecules behave in a certain way, and we've done it using a very simple concept."
In their research, the scientists at IOCB Prague used several unique programs that can calculate how electrons in a molecule behave in the aforesaid higher excited states. Little is known about these states in general, so the work is also groundbreaking because it opens the door to their further study. Moreover, the article published in JACS is not only computational but also experimental.
Researchers from Tomáš Slanina's group supported their findings with an experiment that accurately confirmed the correctness of the calculated data. They also collaborated with one of the world's most respected authorities in the field of (anti)aromatic molecules, Prof. Henrik Ottosson of Uppsala University in Sweden. And this is the second time JACS has taken an interest in their collaboration; the first time was in relation to research on another primary molecule—benzene.
Yet the story of azulene is even more layered. It concerns not only photochemistry but also medicine. Like the first area, the second also bears the seal of IOCB Prague—one of the first drugs developed in its laboratories was an ointment based on chamomile oil containing a derivative of azulene.
Over the decades, the little box labeled Dermazulen, which contains a preparation with healing and anti-inflammatory effects, has found its place in first-aid kits throughout the country.
More information: David Dunlop et al, Excited-State (Anti)Aromaticity Explains Why Azulene Disobeys Kasha's Rule, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c07625
Journal information: Journal of the American Chemical Society