This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Insights into ethylene copolymerization with linear and end-cyclized olefins using a metallocene catalyst
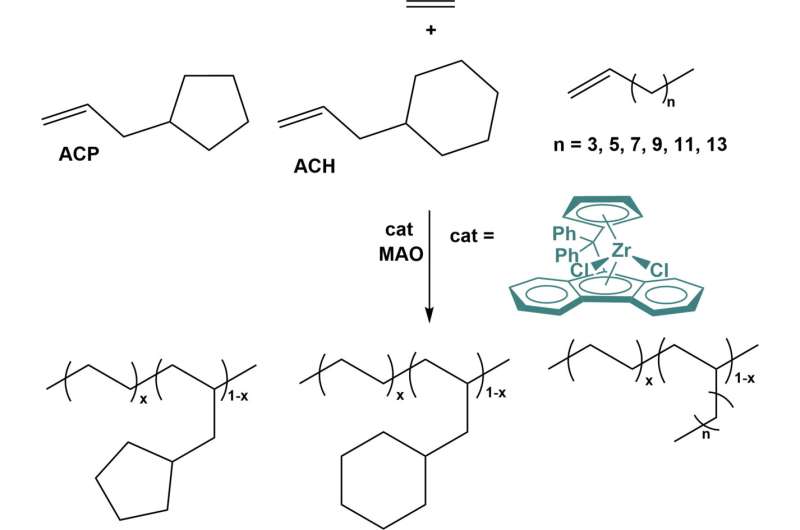
A research team led by Changjiang Wu at SINOPEC (Beijing) Research Institute of Chemical Industry Co., Ltd. in China has made important progress in understanding the polymerization behavior and thermal properties of copolymers formed through ethylene copolymerization with linear and end-cyclized olefins. The findings, published in the journal Engineering, shed light on the potential of utilizing different comonomers in olefin solution polymerization to obtain high-performance polyolefin materials.
Polyolefin elastomers (POEs) are widely used in various industries due to their exceptional properties. However, the high cost associated with their production limits the use of linear α-olefins, such as 1-butene, 1-hexene, and 1-octene, as comonomers in solution polymerization. This research explores the effects of incorporating comonomers with different structures and the resulting properties of the copolymers.
The study reveals that copolymerization of ethylene with linear α-olefins exhibits a higher turn-over frequency (TOF) but lower incorporation compared to copolymerization with end-cyclized α-olefins. This suggests that end-cyclized α-olefins have a higher coordination probability and lower insertion rate.
The copolymerization process leads to the random distribution of comonomers in the polymer chain, effectively disrupting crystallization. Furthermore, end-cyclized α-olefins demonstrate a much stronger crystallization destructive capacity (CDC) in the copolymer than linear α-olefins. This difference may be attributed to the distinct directions of action—linear α-olefins primarily act radially on the main chain, while end-cyclized α-olefins act axially.
The research team also observed that longer linear α-olefins exhibit lower copolymerization efficiency, as indicated by the ratio of incorporation to feeding. In contrast, end-cyclized olefins exhibit high efficiency, potentially due to their behavior resembling a "large methyl group." The unique characteristics of end-cyclized α-olefins, such as increased exposure of the double bond and stronger electronic effects, enhance the coordination probability with the active site of the catalyst.
However, the stability of the active site may hinder further insertion reactions, resulting in a lower insertion rate compared to linear α-olefins. Additionally, the presence of a cyclic substituent near the propagation chain introduces steric hindrance, affecting chain growth.
The microstructure of the resulting copolymers was characterized using three parameters: the product of the reactive ratios (rErX, where the subscript X represents ACP or ACH, and the subscript E represents ethylene), relative monomer dispersity (RMD), and the distribution of the comonomer in a polymer chain ([XX]/[X]). The analysis revealed that all copolymers exhibited a random distribution of comonomers, with most of them isolated within the polymer chain.
The incorporation of comonomers also disrupted crystallization, with longer linear α-olefins demonstrating stronger CDC compared to shorter ones. Notably, end-cyclized α-olefins exhibited a much stronger CDC than linear α-olefins, which can be attributed to their distinct modes of action.
This research provides valuable insights into the copolymerization of ethylene with different α-olefins, highlighting the potential for tailoring the properties of polyolefin materials through the choice of comonomers. The findings contribute to the development of high-performance polyolefin elastomers and expand the possibilities for their industrial applications.
More information: Changjiang Wu et al, Ethylene Copolymerization with Linear and End-Cyclized Olefins via a Metallocene Catalyst: Polymerization Behavior and Thermal Properties of Copolymers, Engineering (2023). DOI: 10.1016/j.eng.2023.07.001
Provided by Engineering