This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Using harmless light to change azobenzene molecules with new supramolecular complex
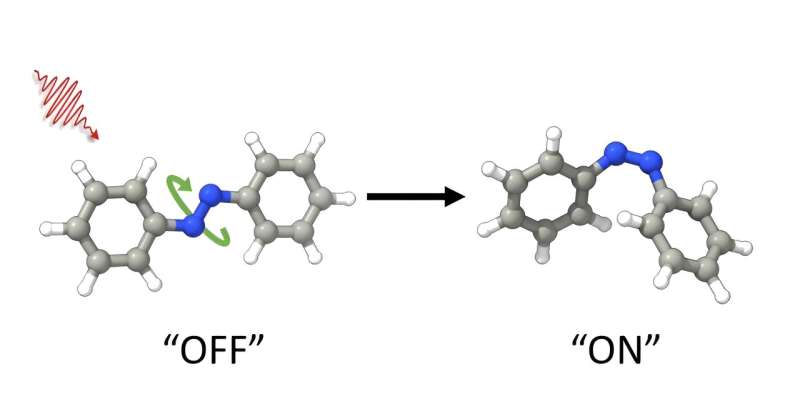
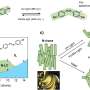
Azobenzenes are incredibly versatile and have many potential uses, such as in making tiny machines and improving technology as well as making light controllable drugs. This molecule can switch between two different forms by light. However, the two forms are in equilibrium, which means that a mixture present that prevents optimal use for applications.
Being able to control them with visible light and enrich only one form opens up new possibilities for these applications, making them more efficient and accessible.
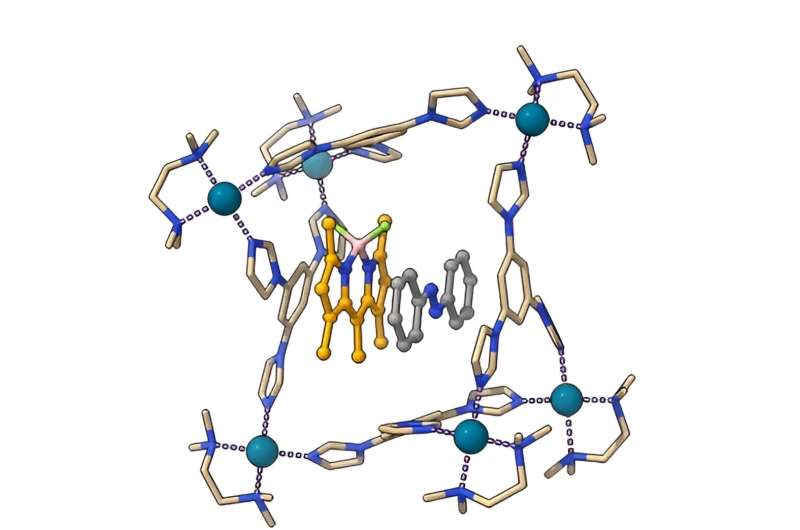
Azobenzenes have traditionally required ultraviolet light for photoisomerization. However, a new approach promises to change the game. Profs. Igor Schapiro from Hebrew University of Jerusalem, Rafal Klajn from the Weizmann Institute of Science and Institute of Science and Technology Austria, and Arri Priimagi from Tampere University along with a team of researchers, have introduced a novel concept termed "disequilibration by sensitization under confinement" or DESC.
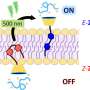
This innovative approach offers for the first time the possibility of prompting a specific molecular transformation, specifically the conversion from an "E" to a "Z" state. They can be thought of as an "on" and "off" form in applications. This change can be triggered by visible light, including wavelengths in the red part of the visible spectrum.
The study, titled "Disequilibrating azobenzenes by visible-light sensitization under confinement," was published in the journal, Science.
What makes azobenzene molecules particularly interesting is their ability to undergo changes in their shape in response to specific types of light, including ultraviolet and visible light. This phenomenon, known as photoisomerization, enables azobenzenes to transition between two distinct shapes or isomers, the "E" and "Z" isomers. This unique attribute holds immense significance, as it unlocks a diverse spectrum of applications spanning nanotechnology, data storage, drug delivery, materials science, and biological research.
In essence, azobenzenes serve as pivotal elements in numerous scientific and technological advancements.
"Through our computational studies and quantum chemical calculations, we've illuminated the path to an innovative approach, which not only advances the fundamental field of azobenzene but also paves the way for practical applications. These applications harness the power of visible light, including red wavelength of light," said Prof. Igor Schapiro, Hebrew University of Jerusalem
Azobenzenes are pivotal components in a wide range of technologies, from molecular switches and actuators to data storage and drug delivery systems. Until now, their photoisomerization necessitated ultraviolet light, limiting their applicability. However, DESC represents a significant breakthrough, offering a supramolecular approach that enables controlled E-to-Z isomerization with harmless light.
This pioneering research opens up exciting new avenues for the utilization of azobenzenes across various fields. By broadening the range of light wavelengths that can induce isomerization, DESC promises to enhance the efficiency and applicability of azobenzene-based technologies.
More information: Julius Gemen et al, Disequilibrating azobenzenes by visible-light sensitization under confinement, Science (2023). DOI: 10.1126/science.adh9059. www.science.org/doi/10.1126/science.adh9059
Journal information: Science
Provided by Hebrew University of Jerusalem