This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
'Crappier-than-crap' molecule exposed in overhaul of carbon-nitrogen-hydrogen chemistry
Russian researchers have explained why scientists studying crude oil and celestial chemistry—as well as certain down-to-earth matters—frequently come across some molecules incorporating carbon, nitrogen, and hydrogen but not other combinations of these three elements. The discovery transforms what used to be a jumble of haphazard rules of organic chemistry into a neat self-contained logical system based on the current fundamental understanding of quantum physics.
Published in The Journal of Physical Chemistry Letters, the study is not only sure to please many a perfectionist out there, but will actually guide astrophysicists toward new "chemical species" in outer space—yes, that's really what they're called.
"If you think about how organic chemistry is often taught, it's a bit like trying to memorize the yellow pages," said Skoltech Professor Artem R. Oganov, the principal investigator of the study. "Some molecules are stable and therefore common, others not so much. Some react readily, others not. But why? There are rules to this that sort of work, but it's not a neat system built from the ground up—more like a disorganized collection of observations."
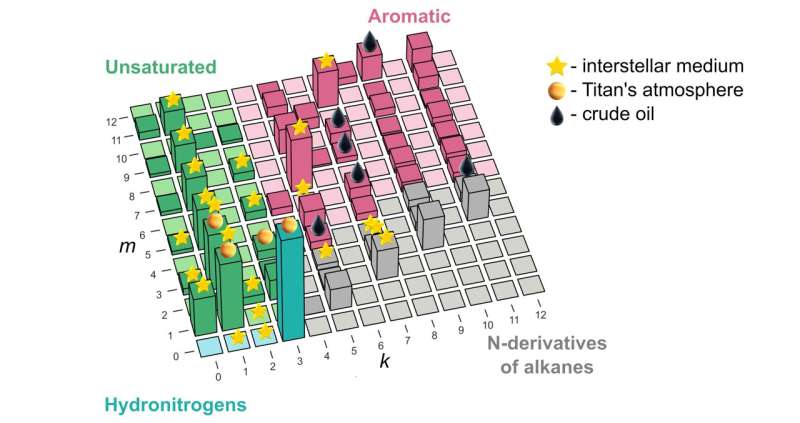
"Our study remedies this situation and shows how to explain and predict these things from first principles for the system of carbon, nitrogen, and hydrogen," he went on. "Now there's one small chart that explains whatever we've seen so far in space or in crude oil, and then some, as far as the combinations of these three atoms are concerned." The underlying principle was borrowed from nuclear physics and nanoscience, and identifies "magic" molecules—those that have lower energy than the molecules of nearest compositions.
The laws of physics say that matter tends to assume the lowest energy state available. That means the pretty, highly organized systems, which someone has put a lot of energy into, tend to degrade when left to their own devices (sorry, parents). Food is a good example: Before too long, a juicy apple packed with tasty energy loses it either to the environment by decaying or, hopefully, to someone who can appreciate it.
Stripped of its energy, the compounds in the apple transform into the familiar reaction products, with the most noticeable molecule among them—think smell—being skatole, named after the Ancient Greek word for poo. So, technically, what makes a molecule crappy is just how low the energy of the molecule that came before was prepared to fall in the course of the reaction.
Skatole (C9NH9) is close to being a "magic" molecule (and another molecule with the same composition and a slightly different arrangement of atoms proved to be magic). Ironically, the malodorous skatole is used in ice-cream and perfume, but in very tiny quantities—guess what for? For its aroma.
While the lead author of the paper, Skoltech master's student Elizaveta Vaneeva from Oganov's Material Discovery Laboratory, was reluctant to delve into the whole skatole aspect of the study, she generously commented on where the new compound stability map might actually be used in science and industry. "The predictions of our model agree well with the list of molecules found in crude oil and outer space.
"But while oil has been thoroughly investigated, when it comes to the interstellar medium and planetary atmospheres, we might be able to give astrochemists some hints about what to look for, and it's much faster and easier to discover new molecules when you have a list of candidates at hand."
"As for the technological applications, one could imagine organic chemists trying to synthesize an industrially useful compound that belongs to the class we were looking at," Vaneeva added. "This could be an organic dye, for example, a blue pigment. And instead of tediously doing experiments to figure out which compound would be stable, they could use our method, which relies on fundamental quantum chemical calculations, to predict the likely candidates. The method has no analogs, is quite fast, and now its predictions have been tested against the published astro- and petrochemistry data."
In the future, the researchers intend to expand their approach to encompass other organic systems, such as amino acids—the building blocks of DNA and RNA—and proteins.
More information: Elizaveta E. Vaneeva et al, Prediction and Rationalization of Abundant C–N–H Molecules in Different Environments, The Journal of Physical Chemistry Letters (2023). DOI: 10.1021/acs.jpclett.3c01753
Journal information: Journal of Physical Chemistry Letters
Provided by Skolkovo Institute of Science and Technology