September 27, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Structural biology—plastic degradation by using wax worm saliva
Plastic waste management is a pressing ecological, social, and economic challenge that has looked to diverse chemical-biology strategies to facilitate biodegradation. In a new report now on Science Advances, Mercedes Spinola-Amilibia and a research team in structural and chemical biology, molecular biology, and microbial biology, in Spain, used the saliva of the lepidopteran Galleria mellonella larvae, to oxidize and depolymerize polyethylene within hours at room temperature.
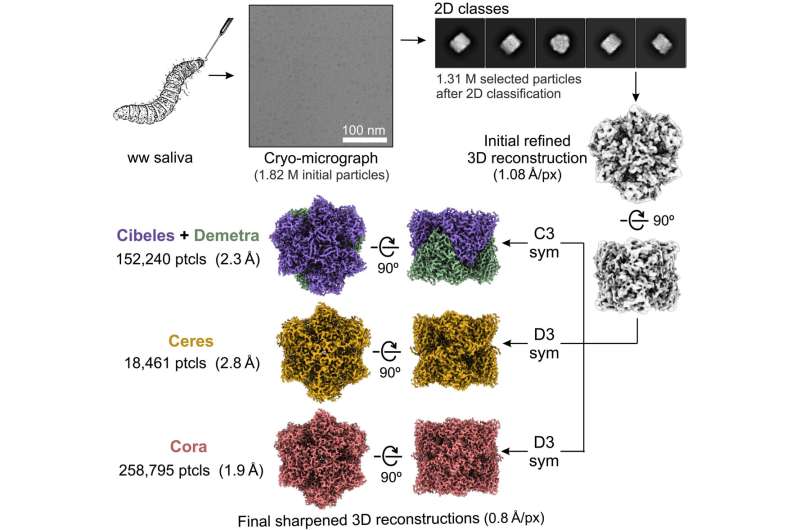
Using cryo-electron microscopy (cryo-EM) the team analyzed the saliva of the microorganisms directly from the native source. Based on 3D reconstructions, they revealed the composition of the buccal secretions to belong to four hexamerins that can oxidize and degrade polyethylene.
Using cryo-EM data and X-ray analysis, they showed the proteins self-assemble into three macromolecular complexes with distinct structural differences to regulate their activity. The results indicated the possibilities of exploring the functionalities of hexamerins for biotechnological functions in vivo.
Experiments to identify the salivary protein composition—Demetra, Cibeles, Ceres and Cora
Using cryo-EM analysis, Spinola-Amilibia and colleagues first revealed the molecular organization and composition of the primary proteins found in the lepidopteran saliva. Mass spectroscopy data revealed the fraction to contain a mix of proteins belonging to the hexamerin/ phenoloxidase (PO) superfamily.
Due to the high molecular mass of the complexes formed by this protein type, the team directly analyzed the buccal secretions using cryo-EM as a first step to explore the 3D architecture and nature of plastic degradation. The results showed clean particles with good contrast. Sequencing based on the cryo-EM maps revealed four sub-populations made of different proteins, however, since the proteins were difficult to identify and their catalyst nature also yet unknown, they were named according to preceding work.
The biologists named the first subpopulation of proteins as Demetra and Cibeles. The second group corresponded to the hexamerin Ceres and they named the third reconstruction Cora. The team showed the possibility of two of these hexamers to oxidize and degrade polyethylene. Incidentally, all four proteins shared a substantial sequence similarity and belong to the hexamerin family, the sequence of the family active site is not conserved.
The self-association of the mature forms of Demetra and Cibeles with structural analysis of Ceres and Cora
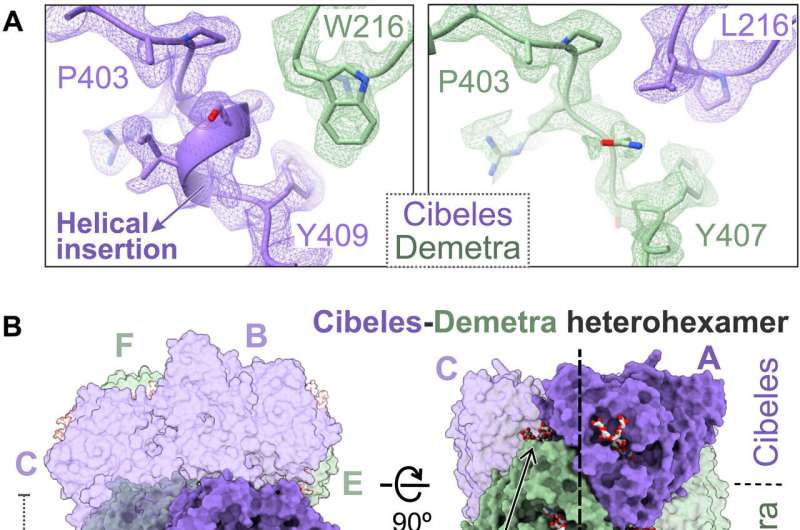
The team attempted to conduct initial 3D classifications of the proteins, although this was not easy due to the heterogeneity that required additional processing. Upon close inspection of the atomic model, the scientists revealed complex molecular interactions with Cibeles and Demetra dimers.
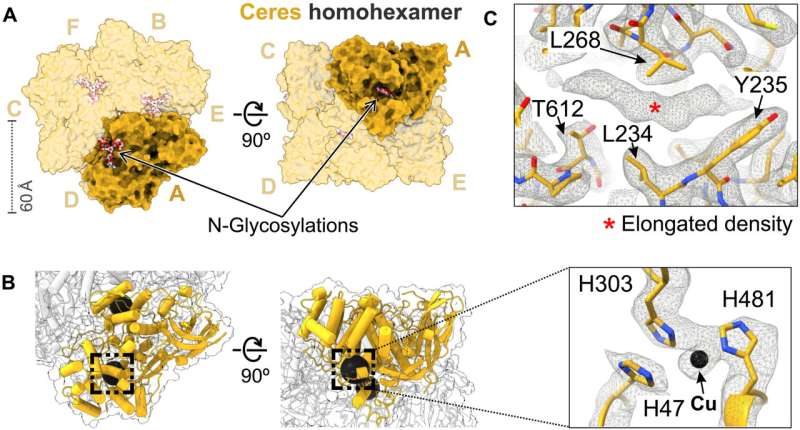
The outcomes emphasized the capacity for Demetra to associate with Cibeles in G. mellonella saliva to form a trimer of heterodimers. The scientists next explored Ceres as a metal-binding hexamerin; a protein identified in saliva with polyethylene-degrading activity. Ceres shared a similar architecture with Demetra and Cibeles, although it self-assorted into homohexamers, and functioned as a glycosylated metal-binding hexamerin, with capacity to engage with organic molecules. Cora on the other hand is a methionine-rich hexamerin that's most abundant in the protein of lepidopteran saliva, proven with cryo-EM particles.
Degrading polyethylene
Biochemists had previously shown the capacity of Demetra and Ceres to degrade polyethylene into different degrees. For instance, when they used 5 μL of the purified recombinant protein Cora to test its capacity to oxidize polyethylene, they noted the polymer-plastic oxidation by using confocal Raman microscopy or spectroscopy. Inactivated Cora did not modify the PE film, Cora effectively degraded polyethylene alongside Demetra and Ceres.
Outlook
In this way, Mercedes Spinola-Amilibia and colleagues highlighted the composition and molecular organization of the primary precursors found in the saliva of the lepidopteran G. mellonella. Using cryo-EM analyses of the native sample, the team achieved valuable information.
Using 3D reconstructions, they revealed the buccal secretions to contain a mixture of four highly related proteins in different proportions and notable structural features named Demetra, Cibeles, Ceres, and Cora, of which the Demetra, Ceres, and Cora triad showed ample capacity to degrade polyethylene.
The work highlighted polyethylene degrading activity to correspond to functional plasticity with biotechnological potential of the hexameric factors found in the wax worm saliva. These factors shed new light for broader applications of biological systems to manage plastic waste beyond the existing capacity of biodegradation by microorganisms.
The study primarily detailed the molecular structure of the compounds towards the development of an emerging field in plastic biodegradability. Researchers aim to combine analyses of X-ray crystallography and cryo-EM to reveal a rich and complex landscape with a group of invertebrate proteins with capacity to receive greater attention in the life sciences. Future investigations will unveil the evolutionary basis underlying the protein functionality to incorporate highly efficient native enzyme-induced plastic recycling and plastic degrading efforts.
More information: Mercedes Spínola-Amilibia et al, Plastic degradation by insect hexamerins: Near-atomic resolution structures of the polyethylene-degrading proteins from the wax worm saliva, Science Advances (2023). DOI: 10.1126/sciadv.adi6813
Jeannette M. Garcia et al, The future of plastics recycling, Science (2017). DOI: 10.1126/science.aaq0324
Journal information: Science Advances , Science
© 2023 Science X Network




















