This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
An escape signal for the nematode: Artificial intelligence helps elucidate structure of a novel light sensor
In a compost heap, the nematode Caenorhabditis elegans finds a richly laid table: at a length of just one millimeter, the worm feeds on bacteria that decompose organic material. It is essential that the animal avoids sunlight—and not just to ensure its body remains at an optimal temperature and does not dry out.
Energy-rich blue and UV light can result in great damage to the cells of the transparent worm, causing the hereditary molecule DNA to mutate, or resulting in the formation of reactive oxygen species such as hydrogen peroxide (H2O2). The latter can, for example, prevent the correct production of proteins and drive cells to death. Laboratory observations show that Caenorhabditis elegans reflexively withdraws from a beam of light.
The nematode does not have eyes, but some of its sensory neurons contain the protein LITE-1, which converts light sensation into biochemical signals in a hitherto unknown manner, ultimately triggering the withdrawal reflex. A group of scientists led by Prof. Alexander Gottschalk of Goethe University Frankfurt, Prof. Gerhard Hummer of the Max Planck Institute of Biophysics and Goethe University, and Dr. Sonya Hanson of the Flatiron Institute has now elucidated the structure and function of LITE-1.
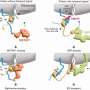
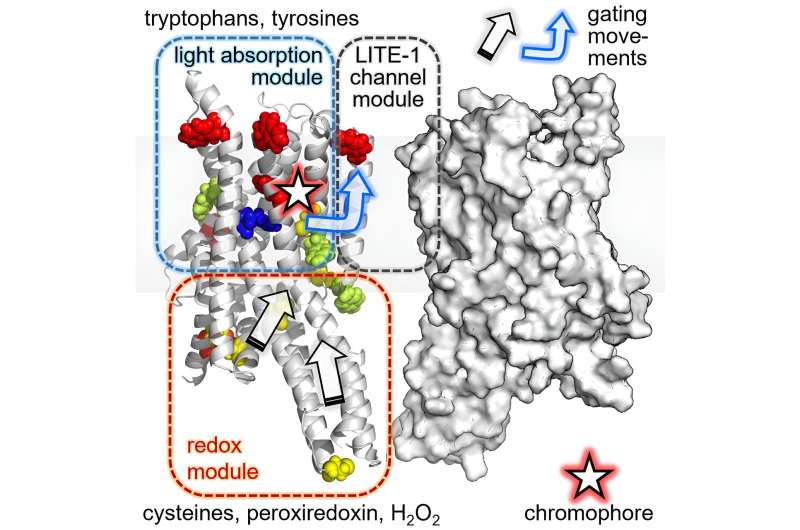
To do so, they used the "AlphaFold2-Multimer" software, an artificial intelligence capable of predicting the structure of proteins and protein complexes based on the sequence of their amino acid building blocks. Their finding: LITE-1 is a so-called channel protein, which is located in the cell membrane and forms a kind of pore through which charged particles—i.e. ions—can pass to cross the membrane.
"The AI worked really well and suggested a plausible structure for LITE-1," says Alexander Gottschalk. "In ensuing genetic experiments, we went on to check whether predictions based on this structure could also be verified in the live nematode and its response to light." To do so, the researchers specifically mutated individual amino acids in LITE-1 and observed the consequences on the light-evoked behavior.
They found that, among other things, the replacement of amino acids that form the channel resulted in a complete loss of function of LITE-1. Additional mutation experiments revealed sites where the protein could interact with H2O2 and also uncovered a central amino acid that appears to be responsible for absorbing the energy generated by UV light.
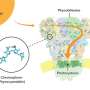
Gerhard Hummer explains: "It appears as if LITE-1 contains a whole network of amino acids, aligned like antennas, to capture the energy of the UV photons and pass it on to a central position in the protein. Here, a cavity is located which in turn could serve as a binding pocket for a chromophore—i.e., a molecule that can absorb photons or their energy."
The researchers' model posits that this as yet unknown chromophore is additionally stimulated directly by blue light, and then transfers all the energy to the LITE-1 protein, leading to the opening of the ion channel and the influx of ions into the cell. The higher ion concentration becomes the starting point for a biochemical-electrical signal that eventually triggers the recoil reflex.
Alexander Gottschalk adds that it apparently plays a role whether H2O2 induced by light exposure in the cells is also present: "The additional activation of LITE-1 by H2O2 ensures that the recoil reflex is not triggered by weak light, only by very intense, tissue-damaging light, such as direct sunlight."
LITE-1 constitutes a very simple form of light perception. Gottschalk says comparisons with insect olfactory receptors suggest that LITE-1 is derived from such an olfactory receptor, which may have coincidentally bound a molecule that could also absorb light and thus transmit a warning signal of harmful light to the animal.
Gottschalk emphasizes the importance of this receptor for the research field of optogenetics, which was co-founded in Frankfurt following the discovery and specification of the first light-dependent ion channel, termed "channelrhodopsin." The field of optogenetics provides the possibility of using light-controlled switches in cells to study cellular functions.
"Both LITE-1 and similar proteins we analyzed may be used as new optogenetic tools, allowing us to extend the spectrum into the UV range." Computational biophysicist Sonya Hanson sees great potential for the future in the research methodology: "The AI we used is now so good that without laborious biochemical work we can still get an idea of how a particular protein works."
More information: Sonya M. Hanson et al, Structure-function analysis suggests that the photoreceptor LITE-1 is a light-activated ion channel, Current Biology (2023). DOI: 10.1016/j.cub.2023.07.008
Journal information: Current Biology
Provided by Goethe University Frankfurt am Main