This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Observing microscopic transformations of electrocatalyst surfaces
Developing technologies that can convert CO2 into synthetic fuels and base chemicals is of key importance for reaching climate goals. The electrochemical reduction of CO2 at copper electrodes using electric power from renewable sources could be used for the production of e-fuels. New studies show that this process changes the arrangement of the copper atoms at the catalyst surface.
Copper is an indispensable catalyst material for CO2 reduction, especially for the synthesis of particular valuable chemicals and fuels, such as ethanol. It is highly favorable if the copper atoms at the surface have a strongly disordered arrangement, and can be achieved, for example, by pre-oxidizing the copper surface or through alloying.
A joint study of the Institute of Experimental and Applied Physics of Kiel University and the Department of Interface Science at the Fritz Haber Institute (FHI) of the Max Planck Society (MPG) showed that such disordered structures also formed spontaneously in the very initial stages of the electrocatalytic CO2 reduction reaction.
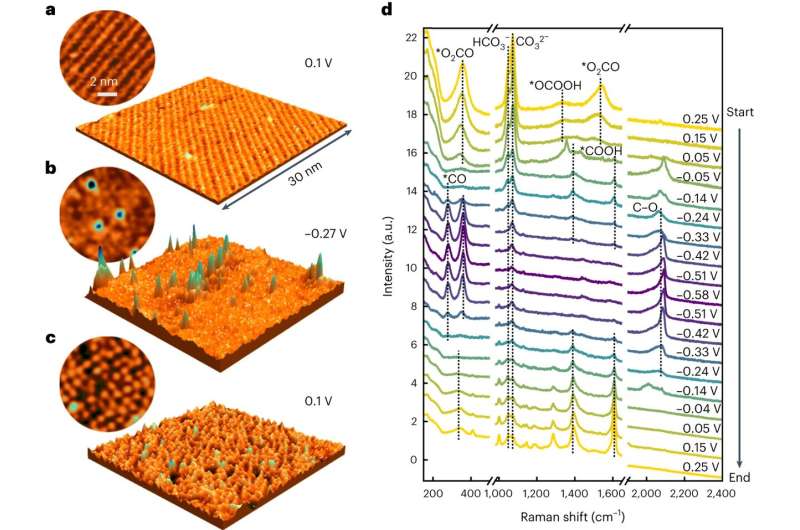
The researchers observed that copper atoms inside the metal moved toward the surface and assembled into a freestanding group of only a few atoms. This transformation of the metal was caused by CO, an intermediate product of the reaction, and was preserved even at high reaction rates. The results were published today in the journal Nature Catalysis.
"Because the electrocatalytic CO2 reduction occurs at metals in aqueous carbonate solution similar to mineral water, and is accompanied by the formation of hydrogen gas, detailed investigations of the process on the atomic scale is difficult," says Olaf Magnussen, Professor for Solid State Physics at Kiel University.
The researchers therefore used a combination of methods that can be employed even under these challenging conditions. The team from Kiel observed the transformation of the copper surface directly by high-resolution electrochemical scanning tunneling microscopy. This method allows direct imaging of the atoms and molecules at the surface.
X-ray diffraction studies, performed by scientists from Kiel and Berlin at the PETRA III synchrotron of DESY in Hamburg confirmed this transformation. In addition, these measurements showed that at high reaction rates the freestanding copper atoms were maintained, but no further formation occurred. Molecular spectroscopy at the Fritz Haber Institute finally indicated that the changes were induced by the formed CO.
"The results suggest a drastic transformation of the electrode surface each time that the electric current required for the CO2 reduction is switched on. This was previously unknown, but could play an important role in catalysis," says Professor Beatriz Roldán Cuenya, Director of the Interface Science Department at FHI.
One strategy to influence the structure of the catalyst and thus the type of formed chemical compounds is to operate the electrode with voltage pulses. Indeed, this had been shown already in previous work by the research team. However, the copper there was periodically oxidized by means of electrical energy, which requires the polarity of the electrode to be reversed.
According to the new results, similar effects might be obtained already by simply switching the current on and off. "Overall, the study confirms our hypothesis that not only the electrode material but also the operating conditions and micro-environment are relevant for the realization of this environmentally friendly technology," says the team.
More information: Reihaneh Amirbeigiarab et al, Atomic-scale surface restructuring of copper electrodes under CO2 electroreduction conditions, Nature Catalysis (2023). DOI: 10.1038/s41929-023-01009-z
Journal information: Nature Catalysis
Provided by Christian-Albrechts-Universität zu Kiel