This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers develop framework to guide electrolyte design for advanced batteries
From cellphones to electric vehicles to microgrids, the future is battery powered. The current go-to lithium-ion batteries, however, require hard-to-find, expensive materials, and their liquid electrolytes can make for a volatile product. Solid-state batteries are a safer option that can hold even more energy, but effectively harnessing their structure–performance relationship has remained a complex barrier to creating better batteries.
Now, however, researchers at Tohoku University's Advanced Institute for Materials Research (WPI-AIMR) and Institute for Materials Research in Japan have developed a framework to predict how the structure of solid-state electrolytes can affect the performance of a battery. They published their findings on July 26 in Chemistry of Materials.
"Developing promising energy storage devices is critical to realize a sustainable future," said co-corresponding author Hao Li, associate professor at WPI-AIMR. "Over the past few decades, many attempts to find 'beyond lithium' battery electrolytes have been reported, and, in particular, divalent closo-type complex hydride (CTCH) electrolytes are valuable alternatives to overcome the safety and energy density limitations of lithium-ion technology."
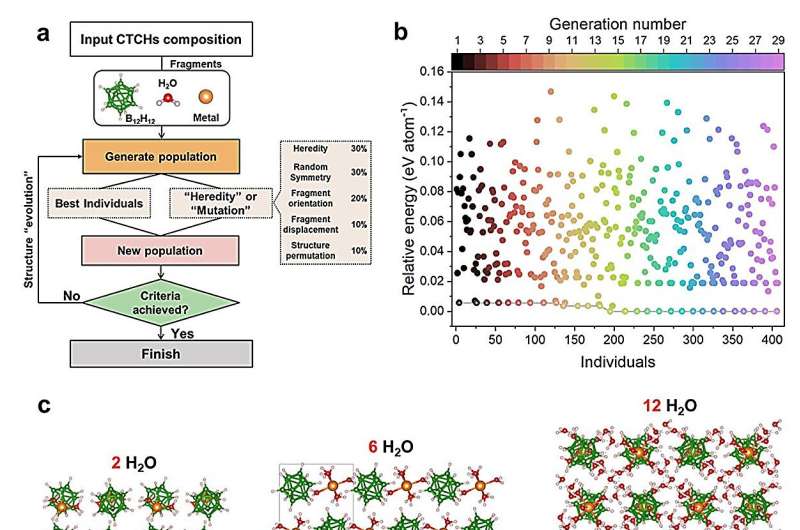
A typical battery consists of oppositely charged metal electrodes in a liquid electrolyte. Lithium ions diffuse the positively charged electrode and attach to carbon on the negatively charged electron. The process reverses when energy is used. CTCH electrolytes, according to the researchers, can accelerate the rate at which positive ions diffuse during the process. This increased conductivity is achieved by adding neutral molecules to the CTCH's structural lattice.
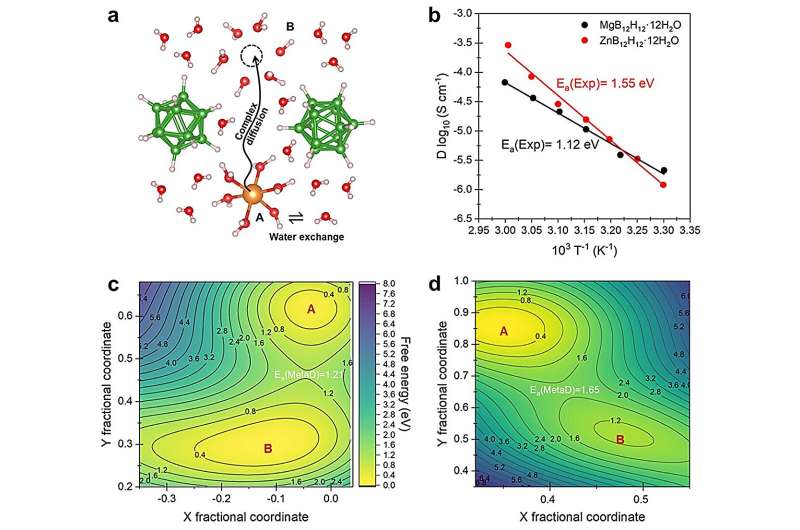
"Neutral molecule-containing CTCHs are a class of promising but highly complicated materials," said co-corresponding author Shin-ichi Orimo, professor and director of WPI-AIMR. "The key determinants of their performance as battery electrolytes and their structure-performance relationships have been a big mystery that hampered the exploration of the ionic diffusion mechanism and the design of high-performance batteries."
To address this challenge, the researchers—a collaboration between the laboratories of Li and Orimo—combined a genetic algorithm, which imitates the process of natural selection to refine a population's subjects, with computational modeling of how energy functions within a system. With this framework, the researchers found they could predict how adding neutral molecules to a CTCH would affect its performance.
Without using any experimental information to set the parameters, the team used this strategy to successfully predict both structural information and diffusion activation energies. Their predictions compared nearly identically with experimental observations.
"Based on these results, we developed robust structure-performance relationships that can precisely predict the divalent CTCH performance and identify the key factors that affect ionic conductivity," Li said. "This study paves a new avenue for building a precise structure-performance picture of complex materials starting from near-zero information."
Next, the researchers said they plan to design and screen high-performance and cost-effective electrolytes, as well as apply the framework to better understand other classes of solid-state electrolytes.
More information: Egon Campos dos Santos et al, Explore the Ionic Conductivity Trends on B12H12 Divalent Closo-Type Complex Hydride Electrolytes, Chemistry of Materials (2023). DOI: 10.1021/acs.chemmater.3c00975
Journal information: Chemistry of Materials
Provided by Tohoku University