This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Researchers demonstrate direct formation of sulfuric acid in the atmosphere without SO2

In the atmosphere, gaseous sulfuric acid can form particles that influence the physical properties of clouds. Thus, the formation of sulfuric acid in the gas phase directly affects the radiative forcing and Earth's climate.
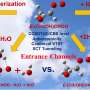
In addition to the known formation from sulfur dioxide, researchers at the Leibniz Institute for Tropospheric Research (TROPOS) have now been able to demonstrate through experiments that there is another formation pathway that has been speculated about for decades. Sulfuric acid in the atmosphere can also be formed directly by the oxidation of organic sulfur compounds.
This new production pathway can be responsible for up to half of the gaseous sulfuric acid formation over the oceans and is thus of high importance for climate projections—especially over the oceans of the Southern Hemisphere, as the researchers write in the journal Nature Communications.
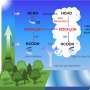
Organic sulfur compounds are mainly emitted from biogenic sources and contribute significantly to Earth's sulfur cycle. The sulfur cycle is important for Earth's climate because the oxidation products of organic sulfur compounds, such as sulfuric acid (H2SO4) and methanesulfonic acid (MSA, CH3SO3H), initiate new particle formation.
Sulfate particles scatter incoming solar radiation efficiently and affect the formation of cloud condensation nuclei (CCN). Number and size of CCN significantly change the microphysical and radiative properties as well as the lifetime of clouds. Hence, knowledge of how H2SO4 is formed in the atmosphere is mandatory to understand fundamental processes in the climate system.
On global scale, with an annual emission rate of about 30 million tons of sulfur, the most important organic sulfur compound is dimethyl sulfide (DMS, CH3SCH3) followed by methyl mercaptan (MeSH, CH3SH) and, to a lesser extent, dimethyl disulfide (DMDS, CH3SSCH3).
Numerous laboratory experiments and model simulations were performed at TROPOS to investigate the atmospheric oxidation pathways of these compounds. "To our knowledge, there is so far no experimental evidence for the direct formation of H2SO4 in the gas phase, except via SO2 oxidation by OH radicals or Criegee intermediates, although this has long been speculated about in the literature and even such pathways are implemented in models," explains Dr. Torsten Berndt of TROPOS.
At TROPOS, experimental proof of direct H2SO4 formation has now been achieved using two flow systems: a horizontal free-jet flow system and a vertical laminar flow tube. With these systems, it was possible to work under different reaction conditions, which allowed an exact investigation of the oxidation processes by using advanced mass spectrometric techniques. In addition, the experimental findings allow the estimation of many kinetic parameters in the sulfur oxidation cycle and thus contribute to an improved understanding of its atmospheric oxidation.
The experimental results were integrated into the complex multiphase chemistry mechanism MCM/CAPRAM existing at TROPOS for model evaluation. "Under the relatively pristine conditions over the Southern oceans, the direct formation of H2SO4 can contribute up to 50% to the total gas phase formation," report Dr. Andreas Tilgner and Dr. Erik H. Hoffmann.
It is postulated that the direct gas-phase formation may be especially important over oceans in the Southern Hemisphere. These regions are often characterized by small SO2-to-DMS ratios, which means that the fraction of this direct production pathway can be important there.
Other atmospheric regions, where small SO2-to-DMS ratios are expected, are in the outflow regimes of marine clouds. There, high H2SO4 concentrations and the formation of new aerosol particles have been already observed, which can now be explained.
More information: Torsten Berndt et al, Direct sulfuric acid formation from the gas-phase oxidation of reduced-sulfur compounds, Nature Communications (2023). DOI: 10.1038/s41467-023-40586-2
Journal information: Nature Communications
Provided by Leibniz-Institut für Troposphärenforschung e. V.