This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
New and improved bioink to enhance 3D bioprinted skeletal muscle constructs
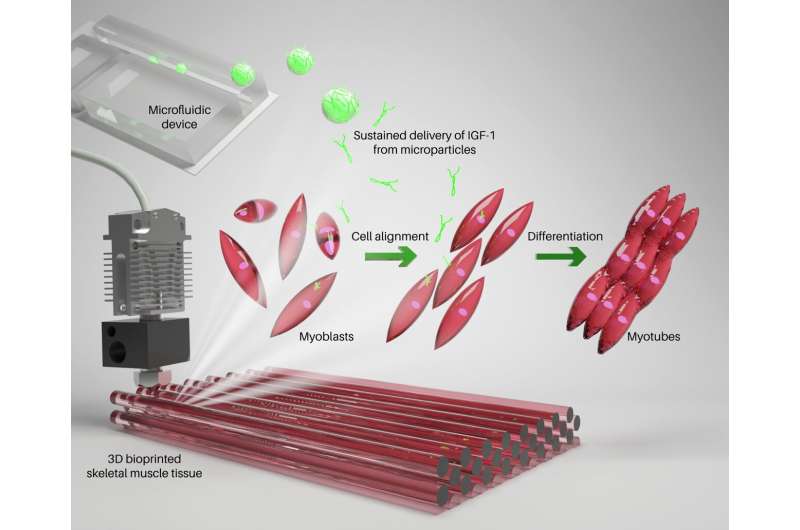
An advancement in 3D bioprinting of native-like skeletal muscle tissues has been made by scientists at the Terasaki Institute for Biomedical Innovation (TIBI). The key to the TIBI scientists' approach lies in their specially formulated bioink, which contains microparticles engineered for sustained delivery of insulin-like growth factor-1 (IGF-1).
As was shown in their recent paper in Macromolecular Bioscience, sustained delivery of IGF-1 enhances the formation of mature skeletal muscle tissue from muscle precursor cells and facilitates their structural alignment. This increases the efficiency of the regenerative process and can lead to successful therapies for people suffering from muscle loss or injury.
The loss of skeletal muscle due to trauma, disease, or surgical procedures results not only in functional impairment but causes damage to associated tissues, such as blood vessels and other structural tissues. Current treatment for such muscle loss is the transfer of a patient's healthy muscle tissue from a different site to the injury site. However, insufficient innervation and other complications with the transplanted tissue can hamper full muscle recovery.
The normal process for muscle development is gradual, where round muscle precursor cells called myoblasts fuse to form tubular-shaped cells called myotubes. These myotubes eventually develop into mature muscle fibers. In addition to muscle cell maturation, precise cellular alignment and orientation are essential to successful muscle contraction and function.
Efforts have been made to bioengineer functional skeletal muscle tissue, but most approaches present their own set of challenges. For example, attempts to engineer native-like skeletal muscle tissue using electrospinning methods have produced muscle tissue with the proper structural alignment and orientation for repair and regeneration; however, the capabilities of the tissues for cell maturation and muscle contraction have proven insufficient.
The TIBI approach utilizes 3D bioprinting with a bioink composed of GelMA (a biocompatible gelatin-based hydrogel), myoblast cells, and microparticles engineered for sustained delivery of IGF-1.
IGF-1 promotes muscle regeneration and repair when present for at least ten days. To provide sustained release of IGF-1 for several days, the researchers used a microfluidic system to fabricate uniformly sized microparticles which were coated with IGF-1. The IGF-1 was gradually released from the surface of the microparticles as the particles degraded.
One week after the muscle constructs were created with the new bioink, the researchers observed enhanced myoblast alignment, fusion, and differentiation into myotubes, which were also shown to grow and elongate significantly more than constructs without a sustained release of IGF-1. Interestingly, ten days after bioprinting, the muscle tissue constructs having sustained release of IGF-1 began to contract spontaneously.
Preclinical studies were carried out with mice receiving implants of 3D bioprinted muscle tissue constructs. Those mice implanted with muscle tissue constructs that offered sustained release of IGF-1 exhibited the highest degree of muscle tissue regeneration six weeks after implantation.
Additional in vivo experiments revealed that sustained release of IGF-1 also triggered a well-regulated inflammatory response that proved beneficial for tissue repair.
"The sustained release of IGF-1 facilitates the maturation and alignment of muscle cells, which is a crucial step in muscle tissue repair and regeneration," said TIBI's Director and CEO, Ali Khademhosseini, Ph.D. "There is great potential for using this strategy for the therapeutic creation of functional, contractile muscle tissue."
More information: Natan Roberto de Barros et al, Enhanced Maturation of 3D Bioprinted Skeletal Muscle Tissue Constructs Encapsulating Soluble Factor‐Releasing Microparticles, Macromolecular Bioscience (2023). DOI: 10.1002/mabi.202300276
Provided by Terasaki Institute for Biomedical Innovation