This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
How rhomboid protease activity is regulated
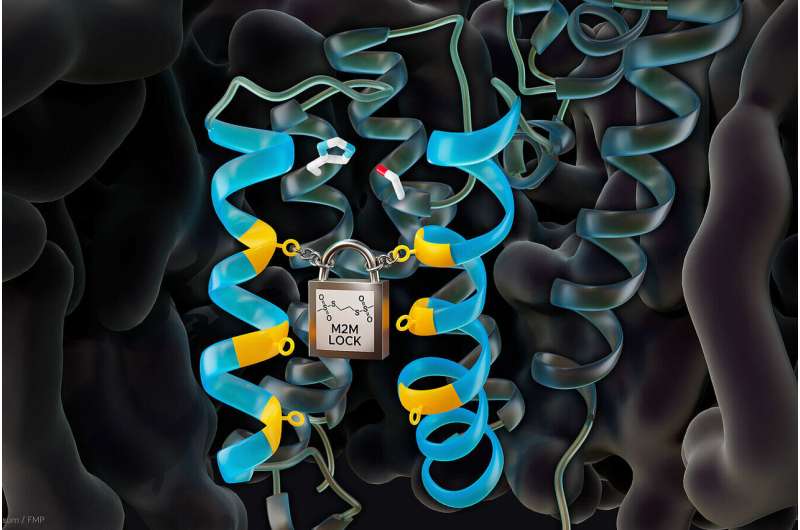
Rhomboid proteases are a promising target for new drugs. Now researchers from the Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) have uncovered a mechanism for regulating enzyme activity. The key role is played by dynamics of the gate discovered a few years ago, which opens briefly when other proteins are cleaved.
The gates are located in the cell membrane and cleave other proteins, triggering a signaling cascade in the cell: as an enzyme, rhomboid proteases are involved in several biological processes in the human body, and play a key role in a number of diseases such as Parkinson's disease, malaria and cancer. Consequently, they are considered to be a promising target for new drugs. Due to their localization, however, these intramembrane proteins are difficult to study.
In 2019, Professor Adam Lange's research group from Berlin's Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) succeeded in producing dynamic images of rhomboid proteases for the first time—using solid-state NMR spectroscopy. In their work, the researchers were able to confirm the conjecture that, to cleave other proteins, a gate opens briefly, enabling substrates to move from the otherwise anhydrous cell membrane to the hydrous active site of the enzyme. As a result, substrates of the now cleaved proteins can detach from the cell membrane and trigger a wide variety of biological processes in the cell.
Demonstration of correlation between gate dynamics and enzyme activity
In their current study within the UniSysCat Cluster of Excellence, FMP researchers have now shown the importance of the gate for the functioning of rhomboid proteases. The findings have recently been published in Science Advances. According to the study, there is a clear correlation between gate dynamics and enzyme activity.
Their current work employed not only solid-state NMR spectroscopy, but also other biophysical methods and biochemical functional assays, as well as molecular dynamics simulations. "This time, to understand how rhomboid proteases function, we combined a whole range of experimental and theoretical techniques and approaches," remarked project leader Adam Lange. "It truly was a highlight of this work."
The researchers used a biophysical model for their experiments. Rhomboid proteases from E. coli bacteria (GlpG)—similar molecules are also found in human mitochondria—were biochemically modified to produce various mutants. These mutants had either a movable or, conversely, a closed gate. If the mutations made it easier for the gate to open, enzyme activity increased; if the gate was closed, activity came to a standstill, causing the substrate to come up against "closed doors," which meant that it could no longer be processed.
Molecular dynamics simulations performed by Professor Han Sun's research group supported and extended the experimental results. "For example, we were able to simulate on the computer exactly how wide the gate has to be open to let substrates through," explained Han Sun.
FMP doctoral student Claudia Bohg, lead author of the current work, is also involved in the search for new compounds, which is taking place in parallel at FMP. "Rhomboid proteases are a clinically important target," she remarked. "The new findings will no doubt help us make significant progress in this area, too."
More information: Claudia Bohg et al, The opening dynamics of the lateral gate regulates the activity of rhomboid proteases, Science Advances (2023). DOI: 10.1126/sciadv.adh3858
Journal information: Science Advances
Provided by Forschungsverbund Berlin e.V. (FVB)